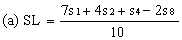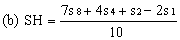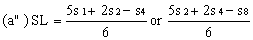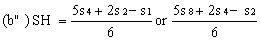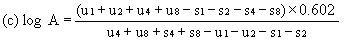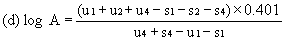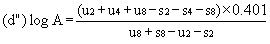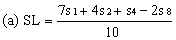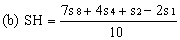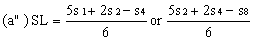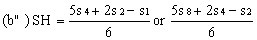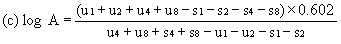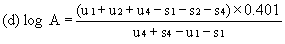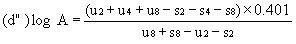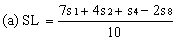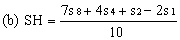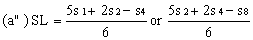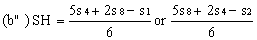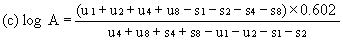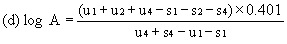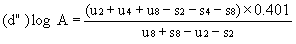S.I. No. 16/1985 - European Communities (Feeding Stuffs) (Methods of Analysis) (Amendment) Regulations. 1985.
S.I. No. 16 of 1985. | ||||||||||
EUROPEAN COMMUNITIES (FEEDING STUFFS) (METHODS OF ANALYSIS) (AMENDMENT) REGULATIONS. 1985. | ||||||||||
I, AUSTIN DEASY, Minister for Agriculture, in exercise of the powers conferred on me by section 3 of the European Communities Act, 1972 (No. 27 of 1972), for the purpose of giving effect to Articles 2 and 3 of Commission Directive No. 84/4/EEC of 20 December, 19831, and Commission Directive No. 84/425/EEC of 25 July, 19842, hereby make the following regulations: 1. (1) These Regulations may be cited as the European Communities (Feeding Stuffs) (Methods of analysis) (Amendment) Regulations, 1985. | ||||||||||
(2) The European Communities (Feeding Stuffs) (Methods of Analysis) Regulations, 1978 to 1982, and these Regulations may be cited together as the European Communities (Feeding Stuffs) (Methods of Analysis) Regulations, 1978 to 1985. 2. Part II of the European Communities (Feeding Stuffs) (Methods of Analysis) Regulations, 1978 ( S.I. No. 250 of 1978 ), is hereby amended by— | ||||||||||
(a) the substitution of the following paragraph for paragraph 7: | ||||||||||
7. Determination of virginiamycin | ||||||||||
—by diffusion in an agar medium— | ||||||||||
1. Purpose and scope | ||||||||||
To determine the virginiamycin content in feedingstuffs and premixes. The lower limit of determination is 2 mg/kg.1 | ||||||||||
2. Principle | ||||||||||
The sample is extracted with a methanolic solution of Tween 80. The extract is decanted or centrifuged and diluted. Its antibiotic activity is determined by measuring the diffusion of virginiamycin in an agar medium inoculated with Micrococcus luteus. Diffusion is shown by the formation of zones of inhibition of the micro-organism. The diameter of these zones is taken to be in direct proportion to the logarithm of the antibiotic concentration over the range of antibiotic concentrations employed. | ||||||||||
1 O.J L15/28. 18 January 1984. | ||||||||||
2 O.J. L238/34, 6 September 1984. | ||||||||||
11 mg virginiamycin is equivalent to 1.000 UK units. | ||||||||||
3. Micro-organism: Micrococcus luteus ATCC 9341 (NCTC 8340, NCIB 8553) | ||||||||||
3.1 Maintenance of stock culture | ||||||||||
Inoculate tubes containing slopes of culture medium (4.1) with Micrococcus luteus and incubate for 24 hours at 30°C. Store the culture in a refrigerator at about 4°C. Reinoculate every two weeks. | ||||||||||
3.2 Preparation of the bacterial suspension(a) | ||||||||||
Harvest the growth from a recently prepared agar slope (3.1) with 2 to 3 ml of sodium chloride solution (4.3). Use this suspension to inoculate 250 ml of culture medium (4.1) contained in a Roux flask and incubate for 18 to 20 hours at 30°C. Harvest the growth in 25 ml of sodium chloride solution (4.3) and mix. Dilute the suspension to 1/10 with sodium chloride solution (4.3). The light transmission of the suspension must be about 75%, measured at 650 nm in a 1 cm cell against sodium chloride solution (4.3). This suspension may be kept for one week at about 4°C. | ||||||||||
4. Culture media and reagents | ||||||||||
4.1 Culture and assay medium(b) | ||||||||||
Meat peptone 6.0 g | ||||||||||
Tryptone 4.0 g | ||||||||||
Yeast extract 3.0 g | ||||||||||
Meat extract 1.5 g | ||||||||||
Glucose 1.0 g | ||||||||||
Agar 10.0 to 20.0g | ||||||||||
Water 1.000 ml | ||||||||||
pH 6.5 (after sterilisation). | ||||||||||
4.2 Phosphate buffer, pH 6 | ||||||||||
Potassium hydrogen phosphate. K2HPO4 2.0 g | ||||||||||
Potassium dihydrogen phosphate. KH2PO4 8.0 g | ||||||||||
Water to 1.000 ml | ||||||||||
4.3 Sodium chloride solution 0.8% (w/v): dissolve 8 g sodium chloride in water and dilute to 1.000 ml; sterilise. | ||||||||||
4.4 Methanol. | ||||||||||
4.5 Mixture of phosphate buffer (4.2) /methanol (4.4): 80/20 (v/v). | ||||||||||
4.6 Tween 80 methanolic solution 0.5% (w/v): dissolve 5 g Tween 80 in methanol (4.4) and dilute to 1,000 ml with methanol. | ||||||||||
4.7 Standard substance: virginiamycin of known activity. | ||||||||||
5. Standard solutions | ||||||||||
Dissolve an accurately weighed quantity of the standard substance (4.7) in methanol (4.4) and dilute with methanol (4.4) to give a stock solution containing 1,000 vg virginiamycin per ml. | ||||||||||
Stored in a stoppered flask at 4°C this solution is stable for up to five days. | ||||||||||
From this stock solution prepare by successive dilution with the mixture (4.5) the following solutions: | ||||||||||
| ||||||||||
| ||||||||||
(a) Other methods may be used provided that it has been established that they give similar bacterial suspensions. | ||||||||||
(b) Any commercial culture medium of similar composition and giving the same results may be used. | ||||||||||
6. Preparation of the extract and assay solutions | ||||||||||
6.1 Extraction | ||||||||||
6.1.1 Products with a virginiamycin content up to 100 mg/kg | ||||||||||
Weigh, to the nearest mg approximately 50 g of sample. Place in a conical flask, add 200 ml of solution (4.6) and shake for 30 minutes. Leave to settle or centrifuge, take 20 ml of the supernatant solution and evaporate to about 5 ml in a rotary evaporator at a temperature not exceeding 40°C. Dilute the residue with the mixture (4.5) to obtain an expected virginiamycin content of 1 vg/ml (=U8). | ||||||||||
6.1.2 Products with a virginiamycin content greater than 100 mg/kg | ||||||||||
Weigh, to the nearest mg, a quantity of sample not exceeding 10.0 g and containing between 1 and 50 mg virginiamycin. Place in a conical flask, add 100 ml of solution (4.6) and shake for 30 minutes. Leave to settle or centrifuge, then dilute the supernatant solution with the mixture (4.5) to obtain an expected virginiamycin content of 1 vg/ml (=U8). | ||||||||||
6.2 Assay solutions | ||||||||||
From solution U8 prepare solutions U4 (expected content: 0.5 vg/ml), U2 (expected content: 0.25 vg/ml) and U1 (expected content: 0.125 vg/ml) by means of successive dilution (1+1) with the mixture (4.5). | ||||||||||
7. Assay procedure | ||||||||||
7. 1 Inoculation of the assay medium | ||||||||||
Inoculate the assay medium (4.1) with the bacterial suspension (3.2) at about 50°C. By preliminary trials on plates with the medium (4.1) determine the quantity of bacterial suspension required to give the largest and clearest zones of inhibition with the various concentrations of virginiamycin. | ||||||||||
7.2 Preparation of the plates | ||||||||||
Diffusion through agar is carried out in plates with the four concentrations of the standard solution (S8, S4, S2 and S1) and the four concentrations of the assay solution (U8, U4, U2 and U1), These four concentrations of extract and standard must necessarily be placed in each plate. To this effect, select plates big enough to allow at least eight holes with a diameter of 10 to 13 mm and not less than 30 mm between centres to be made in the agar medium. The test may be carried out on plates consisting of a sheet of glass with a faced aluminium or plastic ring placed on top, 200 mm in diameter and 20 mm high. | ||||||||||
Pour into the plates a quantity of the medium (4.1) inoculated as in point 7.1, to give a layer about 2mm thick (60 ml for a plate of 200 mm diameter). Allow to set in a level position, bore the holes and place in them exactly measured volumes of assay and standard solutions (between 0.10 and 0.15 ml per hole, according to the diameter). Apply each concentration at least four times so that each determination is subject to an evaluation of 32 zones of inhibition. | ||||||||||
7.3 Incubation | ||||||||||
Incubate the plates for 16 to 18 hours at 30 ± 2°C. | ||||||||||
8. Evaluation | ||||||||||
Measure the diameter of the zones of inhibition to the nearest 0.1 mm. Record the mean measurements for each concentration on semi-logarithmic graph paper showing the logarithm of the concentrations in relation to the diameters of the zones of inhibition. Plot the best fit lines of both the standard solution and the extract, for example as below: | ||||||||||
Determine the "best fit" point for the standard lowest level (SL) using the formula: | ||||||||||
| ||||||||||
Determine the "best fit" point for the standard highest level (SH) using the formula: | ||||||||||
| ||||||||||
Similarly calculate the "best fit" points for the extract lowest level (UH) by substituting u1, u2, u4 and u8 for s1m s2, s4 and s8 in the above formulae1. | ||||||||||
Record the calculated SL and SH values on the same graph paper and join them to give the "best fit" line for the standard solution. Similarly record UL and UH and join them to give the "best fit" line for the extract. | ||||||||||
In the absence of any interference the lines should be parallel. For practical purposes the lines can be considered parallel if the values (SH-SL) and (UH-UL) do not differ by more than 10% from their mean value. | ||||||||||
If the lines are found to be non-parallel either u1 and s1 or u8 and s8 may be discarded and SL, SH, UL and UH calculated, using the alternative formulae, to give alternative "best fit" lines: | ||||||||||
| ||||||||||
| ||||||||||
and similarly for UL and UH. The same criteria of parallelism should be satisfied. The fact that the result has been calculated from three levels should be noted on the final report. | ||||||||||
When the lines are considered as being parallel, calculate the logarithm of the relative activity (log A) by means of one of the following formulae. depending upon whether three or four levels have been used for the assessment of parallelism. | ||||||||||
For four levels | ||||||||||
| ||||||||||
For three levels | ||||||||||
| ||||||||||
or | ||||||||||
| ||||||||||
Activity of sample extract = activity of relevant standard x A | ||||||||||
(U8 = S8 x A) | ||||||||||
1The small letters 's' and 'u' refer to the diameters of the zones of inhibition. | ||||||||||
If the relative activity is found to be outside the range of 0.5 to 2.0 then repeat the assay making appropriate adjustments to the extract concentrations or, if this is not possible, to the standard solutions. When the relative activity cannot be brought into the required range, any result obtained must be considered as approximate and this should be noted on the final report. | ||||||||||
When the lines are considered as not being parallel, repeat the determination. If parallelism is still not achieved, the determination must be considered as unsatisfactory. | ||||||||||
Express the result in milligrams of virginiamycin per kilogram of feedingstuff. | ||||||||||
9. Repeatability | ||||||||||
The difference between the results of two parallel determinations carried out on the same sample by the same analyst should not exceed: | ||||||||||
— 2 mg/kg, in absolute value, for contents of virginiamycin up to 10 mg/kg. | ||||||||||
— 20% relative to the higher value for contents of 10 to 25 mg/kg, | ||||||||||
— 5 mg/kg. in absolute value, for contents of 25 to 50 mg/kg, | ||||||||||
— 10% relative to the higher value for contents above 50 mg/kg. | ||||||||||
(b) the substitution of the following paragraph for paragraph 25 (inserted by Regulation 2 of the European Communities (Feeding Stuffs) (Methods of Analysis) (Amendment) and Methods of Sampling) Regulations, 1980 ( S.I. No. 14 of 1980 )): | ||||||||||
25. Determination of Zinc Bacitracin | ||||||||||
— by diffusion in an agar medium — | ||||||||||
1. Purpose and scope | ||||||||||
To determine the zinc bacitracin content in feedingstuffs and premixes. The lower limit of determination is 5 mg/kg.1 | ||||||||||
2. Principle | ||||||||||
The sample is extracted at pH 2 with a mixture of methanol/water/hydrochloric acid, and a sodium sulphide solution. The addition of sodium sulphide is to precipitate any soluble copper salts that may interfere with the assay. The extract is brought to pH 6. 5. concentrated (where necessary) and diltued. Its antibiotic activity is determined by measuring the diffusion of zinc bacitracin in an agar medium inoculated with Micrococcus luteus (flavus), Diffusion is shown by the formation of zones of inhibition of the micro-organism. The diameter of these zones is taken to he in direct proportion to the logarithm of the antibiotic concentration over the range of antibiotic concentrations employed. | ||||||||||
3. Micro-organism: Micrococcus luteus (flavus) ATCC 10240 (NCTC 7743) | ||||||||||
3.1 Maintenance of stock culture | ||||||||||
Inoculate tubes containing slopes of culture medium (4.1) with Micrococcus luteus (flavus) and incubate for 24 hours at 30°C. Store the culture in a refrigerator at about 4°C. Reinoculate every two weeks. | ||||||||||
3.2 Preparation of the bacterial suspension(a) | ||||||||||
11 mg feedingstuff grade zinc bacitracin is equivalent to 42 international units (i.u.) | ||||||||||
(a) Other methods may be used provided that it has been established that they give similar bacterial suspensions. | ||||||||||
Harvest the growth from a recently prepared agar slope (3.1) with 2 to 3 ml of sodium chloride solution (4.3). Use this suspension to inoculate 250 ml of culture medium (4.1) contained in a Roux flask and incubate for 18 to 20 hours at 30°C. Harvest the growth in 25 ml of sodium chloride solution (4.3) and mix. Dilute the suspension to 1/10 with sodium chloride solution (4.3). The light transmission of the suspension must be about 75%, measured at 650 nm in a 1 cm cell against sodium chloride solution (4.3). This suspension may be kept for one week at about 4°C. | ||||||||||
4. Culture media and reagents | ||||||||||
4.1 Culture medium(b) | ||||||||||
Meat peptone 6.0 g | ||||||||||
Tryptone 4.0 g | ||||||||||
Yeast extract 3.0 g | ||||||||||
Meat extract 1.5 g | ||||||||||
Glucose 1.0 g | ||||||||||
Agar 10.0 to 20.0 g | ||||||||||
Water 1.000 ml | ||||||||||
pH 6.5 to 6.6 (after sterilisation) | ||||||||||
4.2 Assay medium(b) | ||||||||||
Tryptone 10.0 g | ||||||||||
Yeast extract 3.0 g | ||||||||||
Meat extract 1.5g | ||||||||||
Glucose 1.0 g | ||||||||||
Agar 10.0 to 20.0 g | ||||||||||
Tween 801ml | ||||||||||
Water 1,000 ml | ||||||||||
pH 6.5 (after sterilisation) 1.000 ml | ||||||||||
4.3 Sodium chloride solution 0.8% (w/v): dissolve 8 g sodium chloride in water and dilute to 1,000 ml; sterilise. | ||||||||||
4.4 Mixture of methanol/water/hydrochloric acid (4.6): | ||||||||||
80/17. 5/2. 5 (v/v/v). | ||||||||||
4.5 Phosphate buffer, pH 6.5: | ||||||||||
Potassium hydrogen phosphate K2HPO4 22.15 g | ||||||||||
Potassium hydrogen phosphate KH2PO4 27.85 g | ||||||||||
Water to 1,000 ml. | ||||||||||
4.6 Hydrochloric acid (d: 1.18 to 1.19). | ||||||||||
4.7 Hydrochloric acid (0.1 M). | ||||||||||
4.8 Sodium hydroxide 1 M solution. | ||||||||||
4.9 Sodium sulphide about 0.5 M solution. | ||||||||||
4.10 Bromocresol purple solution 0.04% (w/v): dissolve 0.1 g of bromocresol purple in 18.5 ml of 0.01 M sodium hydroxide solution. Make up the volume to 250 ml with water and mix. | ||||||||||
4.11 Standard substance: zinc bacitracin of known activity (in i.u.). | ||||||||||
5. Standard solutions | ||||||||||
Weigh out a quantity of standard zinc bacitracin (4.11) corresponding to 1.050 i.u. (according to the activity indicated), Add 5 ml of (0.1 M hydrochloric acid (4.7) and leave to stand for 15 minutes. Add 30 ml of water, adjust the pH to 4.5 with phosphate buffer (4.5) (about 4 ml), make up to a volume of 50 ml with water and mix well (1 ml = 21 i.u.). | ||||||||||
(b) Any commercial culture medium of similar composition and giving the same results may be used. | ||||||||||
From this solution prepare by successive dilution with phosphate buffer (4.5) the following solutions: | ||||||||||
| ||||||||||
| ||||||||||
6. Preparation of the extract and assay solutions | ||||||||||
6.1 Extraction | ||||||||||
6.1.1 Premixes and mineral feeds | ||||||||||
Weigh, to the nearest mg, 2 to 5 g of sample. Add 29.0 ml of the mixture (4.4) and 1.0 ml of sodium sulphide solution (4.9) and shake briefly. Check that the pH is about 2. Shake for 10 minutes, add 30 ml of phosphate buffer (4.5), shake for 15 minutes and centrifuge. Take a suitable aliquot of the supernatant solution and adjust the pH to 6.5 by means of 1 M hydroxide solution (4.8) with a pH-meter or with the bromocresol purple solution (4.10) as indicator. Dilute with phosphate buffer (4.5) to obtain an expected zinc bacitracin content of 0.42 i.u. /ml (=U8). | ||||||||||
6.1.2 Protein concentrates | ||||||||||
Weigh, to the nearest mg, approximately 10 g of sample. Add 49.0 ml of the mixture (4.4) and 1.0 ml of sodium sulphide solution (4.9) and shake briefly. Check that the pH is about 2. Shake for 10 minutes. Add 50 ml of phosphate buffer (4.5), shake for 15 minutes and centrifuge. Take a suitable volume of the supernatant solution and adjust the pH to 6.5 by means of 1 M sodium hydroxide solution (4.8) with a pH-meter or with the bromocresol purple solution (4.10) as indicator. Evaporate to approximately half volume in a rotary evaporator at a temperature not exceeding 35°C. | ||||||||||
Dilute with phosphate buffer (4.5) to obtain an expected zinc bacitracin content of 0.42 i.u. /ml (= U8). | ||||||||||
6.1.3 Other feeds | ||||||||||
Weigh, to the nearest mg, 10 to 20 g of sample (20g for an expected zinc bacitracin content of 5 mg/kg). Add a mixture of 24.0 ml of the mixture (4.4) and 1.0 ml of sodium sulphide solution (4.9) and homogenise for 10 minutes. Add 25 ml of phosphate buffer (4.5), shake for 15 minutes and centrifuge. Take 20 ml of the supernatant solution and adjust the pH to 6.5 by means of 1 M sodium hydroxide solution (4.8) with a pH-meter or with the bromocresol purple solution (4.10) as indicator. Evaporate to about 4 ml in a rotary evaporator at a temperature not exceeding 35°C. Dilute the residue with phosphate buffer (4.5) to obtain an expected zinc bacitracin content of0.42 i.u. /ml (=U8). | ||||||||||
6.2 Assay solutions | ||||||||||
From solution U8 prepare solutions U4 (expected content: 0.21 i.u. /ml), U2 (expected content 0.105 i.u. /ml) and U1 (expected content: 0.0525 i.u. /ml) by means of successive dilution (1 + 1) with phosphate buffer (4.5). | ||||||||||
7. Assay procedure | ||||||||||
7.1 Inoculation of the assay medium | ||||||||||
Inoculate the assay medium (4.2) with the bacterial suspension (3.2) at about 50°C. By preliminary trials on plates with assay medium (4.2) determine the quantity of bacterial suspension required to give the largest and clearest zones of inhibition with the various concentrations of zinc bacitracin. | ||||||||||
7.2 Preparation of the plates | ||||||||||
Diffusion through agar is carried out in plates with the four concentrations of the standard solution (S8, S4, S2 and S1) and the four concentrations of the assay solution (U8, U4, U2 and U1). These four concentrations of extract and standard must necessarily be placed in each plate. To this effect, select plates big enough to allow at least eight holes with a diameter of 10 to 13 mm and not less than 30 mm between centres to be made in the agar medium. The test may be carried out on plates consisting of a sheet of glass with a faced aluminium or plastic ring placed on top, 200 mm in diameter and 20 mm high. | ||||||||||
Pour into the plates a quantity of the medium (4.2) inoculated as in point 7.1, to give a layer about 2 mm thick (60 ml for a plate of 200 mm diameter). Allow to set in a level position, bore the holes and place in them exactly measured volumes of assay and standard solutions (between 0.10 and 0.15 ml per hole, according to the diameter). Apply each concentration at least four times so that each determination is subject to an evaluation of 32 zones of inhibition. | ||||||||||
7.3 Incubation | ||||||||||
Incubate the plates for 16 to 18 hours at 30 ± 2°C. | ||||||||||
8. Evaluation | ||||||||||
Measure the diameter of the zones of inhibition to the nearest 0.1 mm. Record the mean measurements for each concentration on semi-logarithmic graph paper showing the logarithm of the concentrations in relation to the diameters of the zones of inhibition. Plot the best fit lines of both the standard solution and the extract, for example as below: | ||||||||||
Determine the "best fit" point for the standard lowest level (SL) using the formula: | ||||||||||
| ||||||||||
Determine the "best fit" point for the standard highest level (SH) using the formula: | ||||||||||
| ||||||||||
Similarly calculate the "best fit" points for the extract lowest level (UL) by substituting u1, u2, u4 and u8 for s1, s2, s4 and s8 in the above formulae1. | ||||||||||
Record the calculated SL and SH values on the same graph paper and join them to give the "best fit" line for the standard solution. Similarly record UL and UH and join them to give the "best fit" line for the extract. | ||||||||||
In the absence of any interference the lines should be parallel. For practical purposes the lines can be considered parallel if the values (SH-SL) and (UH-UL) do not differ by more than 10% from their mean value. | ||||||||||
If the lines are found to be non-parallel either u1 and s1 or u8 and s8 may be discarded and SL, SH, UL and UH calculated, using the alternative formulae, to give alternative "best fit" lines: | ||||||||||
| ||||||||||
1The small letters 's' and 'u' refer to the diameters of the zones of inhibition. | ||||||||||
| ||||||||||
and similarly for UL and UH. The same criteria of parallelism should be satisfied. The fact that the result has been calculated from three levels should be noted on the final report. | ||||||||||
When the lines are considered as being parallel, calculate the logarithm of the relative activity (log A) by means of one of the following formulae, depending upon whether three or four levels have been used for the assessment of parallelism. | ||||||||||
For four levels | ||||||||||
| ||||||||||
For three levels | ||||||||||
| ||||||||||
or | ||||||||||
| ||||||||||
Activity of sample extract = activity of relevant standard x A | ||||||||||
(U8 = S8 x A) | ||||||||||
If the relative activity is found to be outside the range of 0.5 to 2.0 then repeat the assay making appropriate adjustments to the extract concentrations or, if this is not possible, to the standard solutions. When the relative activity cannot be brought into the required range, any result obtained must be considered as approximate and this should be noted on the final report. | ||||||||||
When the lines are considered as not being parallel, repeat the determination. If parallelism is still not achieved, the determination must be considered as unsatisfactory. | ||||||||||
Express the result in milligrams of zinc bacitracin base per kilogram of feedingstuff. | ||||||||||
9. Repeatability | ||||||||||
The difference between the results of two parallel determinations carried out on the same sample by the same analyst should not exceed: | ||||||||||
— 2 mg/kg, in absolute value, for contents of zinc bacitracin base up to 10 mg/kg, | ||||||||||
— 20% relative to the higher value for contents from 10 to 25 mg/kg, | ||||||||||
— 5 mg/kg, in absolute value, for contents of 25 to 50 mg/kg, | ||||||||||
— 10% relative to the higher value for contents above 50 mg/kg. | ||||||||||
(c) the insertion of the following paragraph after paragraph 29 (inserted by Regulation 3 (b) of the European Communities (Feeding Stuffs) (Methods of Analysis) (Amendment) Regulations, 1982 ( S.I. No. 261 of 1982 )): | ||||||||||
30. Determination of Spiramycin | ||||||||||
— by diffusion in an agar medium — | ||||||||||
1. Purpose and scope | ||||||||||
To determine the spiramycin content in feedingstuffs and premixes. The lower limit of determination is 1 mg/kg1. | ||||||||||
2. Principle | ||||||||||
The sample is extracted with a mixture of methanol/phosphate-bicarbonate buffer at pH 8. The extract is decanted or centrifuged and diluted. Its antibiotic activity is determined by measuring the diffusion of spiramycin in an agar medium inoculated with Micrococcus luteus. Diffusion is shown by the formation of zones of inhibition of the micro-organism. The diameter of these zones is taken to be in direct proportion to the logarithm of the antibiotic concentration over the range of antibiotic concentrations employed. | ||||||||||
3. Micro-organism: Micrococcus luteus ATCC 9341 (NCTC 8340, NCIB 8553) | ||||||||||
3.1 Maintenance of stock culture | ||||||||||
Inoculate tubes containing slopes of culture medium (4.1) with Micrococcus luteus and incubate for 24 hours at 30°C. Store the culture in a refrigerator at about 4°C. Reinoculate every two weeks. | ||||||||||
3.2 Preparation of the bacterial suspension(a) | ||||||||||
Harvest the growth from a recently prepared agar slope (3.1) with 2 to 3 ml of sodium chloride solution (4.3). Use this suspension to inoculate 250 ml of culture medium (4.1) contained in a Roux flask and incubate for 18 to 20 hours at 30°C. Harvest the growth in 25 ml of sodium chloride solution (4.3) and mix. Dilute the suspension to 1/10 with sodium chloride solution (4.3). The light transmission of the suspension must be about 75%, measured at 650 nm in a 1 cm cell against sodium chloride solution (4.3). This suspension may be kept for one week at about 4°C. | ||||||||||
4. Culture media and reagents | ||||||||||
4.1 Culture medium(b) | ||||||||||
Meat peptone 6.0 g | ||||||||||
Tryptone 4.0 g | ||||||||||
Yeast extract 3.0 g | ||||||||||
Meat extract 1.5 g | ||||||||||
Glucose 1.0 g | ||||||||||
Agar 10.0 to 20.0 g | ||||||||||
Water 1,000 ml | ||||||||||
pH 6.5 to 6.6 (after sterilisation). | ||||||||||
4.2 Assay medium(b) | ||||||||||
Tryptone 5.0 g | ||||||||||
Yeast extract 4.0 g | ||||||||||
Meat extract 3.0 g | ||||||||||
Agar 10.0 to 20.0 g | ||||||||||
Water 1,000 ml | ||||||||||
pH 8.0 (after sterilisation). | ||||||||||
11 mg spiramycin base is equivalent to 3,200 international units (I.U.). | ||||||||||
(a) Other methods may be used provided that it has been established that they give similar bacterial suspensions. | ||||||||||
(b) Any commercial culture medium of similar composition and giving the same results may be used. | ||||||||||
4.3 Sodium chloride solution 0.8% (w/v) | ||||||||||
Dissolve 8 g of sodium chloride in water and dilute to 1,000 ml; sterilise. | ||||||||||
4.4 Phosphate-bicarbonate buffer, pH 8.0 | ||||||||||
Dipotassium hydrogen phosphate K2HPO4 16.7 g | ||||||||||
Potassium dihydrogen phosphate KH2PO4 0.5 g | ||||||||||
Sodium hydrogen carbonate NaHCO3 20.0 g | ||||||||||
Water to 1,000 ml | ||||||||||
4.5 Mixture of methanol phosphate-bicarbonate buffer (4.4) | ||||||||||
50/50 (v/v) | ||||||||||
4.6 Standard substance | ||||||||||
Spiramycin of known activity (in i.u.). | ||||||||||
5. Standard solutions | ||||||||||
Dissolve an accurately weighed quantity of the standard substance (4.6) in the mixture (4.5) and dilute with the same mixture to give a stock solution containing 1,000 i.u. spiramycin per ml. Stored in a stoppered flask at 4°C this solution is stable for up to five days. | ||||||||||
From this stock solution prepare by successive dilution with the mixture (4.5) the following solutions: | ||||||||||
| ||||||||||
| ||||||||||
6. Preparation of the extract and assay solutions | ||||||||||
6.1 Extraction | ||||||||||
6.1.1 Products with a spiramycin content above 2.5 mg/kg | ||||||||||
Weigh, to the nearest mg, 1 to 20 g of sample for premixes and 20 g of the sample for feedingstuffs. Add 100 ml of the mixture (4.5) and shake for 30 minutes. Centrifuge or decant and dilute the supernatant solution with the mixture (4.5) to obtain an expected spiramycin content of 1 i.u./ml (=U8). | ||||||||||
6.1.2 Products with a spiramycin content lower than 2.5 mg/kg | ||||||||||
Weigh, to the nearest mg, approximately 20 g of sample. Add 100 ml of the mixture (4.5) and shake for 30 minutes. Centrifuge for a few minutes, take 50 ml of the supernatant solution and evaporate to about 4 ml under reduced pressure in a rotary evaporator at a temperature not exceeding 40°C. Dilute the residue with the mixture (4.5) to obtain an expected spiramycin content of 1 i.u. /ml (=U8). | ||||||||||
6.2 Assay solutions | ||||||||||
From solution U8 prepare solutions U4 (expected content: 0.5 i.u. /ml), U2 (expected content: 0.25 IU/ml) and U1 (expected content: 0.125 i.u./ml) by means of successive dilution (1+1) with the mixture (4.5). | ||||||||||
7. Assay procedure | ||||||||||
7.1 Inoculation of the assay medium | ||||||||||
Inoculate the assay medium (4.2) with the bacterial suspension (3.2) at about 50°C. By preliminary trials on plates with assay medium (4.2) determine the quantity of bacterial suspension required to give the largest and clearest zones of inhibition with the various concentrations of spiramycin. | ||||||||||
7.2 Preparation of the plates | ||||||||||
Diffusion through agar is carried out in plates with the four concentrations of the standard solution (S8, S4, S2 and S1) and the four concentrations of the assay solution (U8, U4, U2 and U1). These four concentrations of extract and standard must necessarily be placed in each plate. To this effect, select plates big enough to allow at least eight holes with a diameter of 10 to 13 mm and not less than 30 mm between centres to be made in the agar medium. The test may be carried out on plates consisting of a sheet of glass with a faced aluminium or plastic ring placed on top, 200 mm in diameter and 20 mm high. | ||||||||||
Pour into the plates a quantity of the medium (4.1) inoculated as in point 7.1, to give a layer about 2mm thick (60 ml for a plate of 200 mm diameter). Allow to set in a level position, bore the holes and place in them exactly measured volumes of assay and standard solutions (between 0.10 and 0.15 ml per hole, according to the diameter). Apply each concentration at least four times so that each determination is subject to an evaluation of 32 zones of inhibition. | ||||||||||
7.3 Incubation | ||||||||||
Incubate the plates for 16 to 18 hours at 30 ± 2°C. | ||||||||||
8. Evaluation | ||||||||||
Measure the diameter of the zones of inhibition to the nearest 0.1 mm. Record the mean measurements for each concentration on semi-logarithmic graph paper showing the logarithm of the concentrations in relation to the diameters of the zones of inhibition. Plot the best fit lines of both the standard solution and the extract, for example as below: | ||||||||||
Determine the "best fit" point for the standard lowest level (SL) using the formula: | ||||||||||
| ||||||||||
Determine the "best fit" point for the standard highest level (SH) using the formula: | ||||||||||
| ||||||||||
Similarly calculate the "best fit" points for the extract lowest level (UH) by substituting u1, u2, u4 and u8 for s1, s2, S4 and s8 in the above formulae1. | ||||||||||
Record the calculated SL and SH values on the same graph paper and join them to give the "best fit" line for the standard solution. Similarly record UL and UH and join them to give the "best fit" line for the extract. | ||||||||||
In the absence of any interference the lines should be parallel. For practical purposes the lines can be considered parallel if the values (SH-SL) and (UH-UL) do not differ by more than 10% from their mean value. | ||||||||||
If the lines are found to be non-parallel either u1 and s1 or u8 and s8 may be discarded and SL, SH, UL and UH calculated, using the alternative formulae, to give alternative "best fit" lines: | ||||||||||
| ||||||||||
| ||||||||||
1 The small letters 's' and 'u' refer to the diameters of the zones of inhibition. | ||||||||||
and similarly for UL and UH. The same criteria of parallelism should be satisfied. The fact that the result has been calculated from three levels should be noted on the final report. | ||||||||||
When the lines are considered as being parallel, calculate the logarithm of the relative activity (log A) by means of one of the following formulae, depending upon whether three or four levels have been used for the assessment of parallelism. | ||||||||||
For four levels | ||||||||||
| ||||||||||
For three levels | ||||||||||
| ||||||||||
or | ||||||||||
| ||||||||||
Activity of sample extract = activity of relevant standard x A | ||||||||||
(U8 = S8 X A) | ||||||||||
If the relative activity is found to be outside the range of 0.5 to 2.0 then repeat the assay making appropriate adjustments to the extract concentrations or, if this is not possible, to the standard solutions. When the relative activity cannot be brought into the required range, any result obtained must be considered as approximate and this should be noted on the final report. | ||||||||||
When the lines are considered as not being parallel, repeat the determination. If parallelism is still not achieved, the determination must be considered as unsatisfactory. | ||||||||||
Express the result in milligrams of spiramycin base per kilogram of feedingstuff. | ||||||||||
9. Repeatability | ||||||||||
The difference between the results of two parallel determinations carried out on the same sample by the same analyst should not exceed: | ||||||||||
— 2 mg/kg, in absolute value, for contents of spiramycin base up to 10 mg/kg, | ||||||||||
— 20% relative to the higher value for contents of 10 to 25 mg/kg, | ||||||||||
— 5 mg/kg, in absolute value, for contents of 25 to 50 mg/kg, | ||||||||||
— 10% relative to the higher value for contents above 50 mg/kg. | ||||||||||
GIVEN under my Official Seal, this 28th day of January, 1985. | ||||||||||
AUSTIN DEASY | ||||||||||
Minister for Agriculture. | ||||||||||
EXPLANATORY NOTE. | ||||||||||
These Regulations, which implement certain provisions of Commission Directive 84/4/EEC and the provision of Commission Directive 84/425/EEC prescribe: | ||||||||||
( a ) new methods, replacing existing methods, by which analyses of animal feeding stuffs for virginiamycin and zinc bacitracin are to be carried out for the purposes of the European Communities (Feeding Stuffs) (Additives) Regulations, 1974 to 1984; and | ||||||||||
( b ) the method by which analysis of animal feeding stuffs for spiramycin is to be carried out for the purpose of those Regulations. |