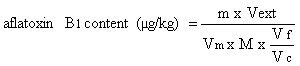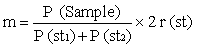S.I. No. 370/1993 - European Communities (Feedingstuffs) (Methods of Analysis) (Amendment) Regulations, 1993.
S.I. No. 370 of 1993. | |||||||||||||||||||||||||||||
EUROPEAN COMMUNITIES (FEEDINGSTUFFS) (METHODS OF ANALYSIS) (AMENDMENT) REGULATIONS, 1993. | |||||||||||||||||||||||||||||
I, JOE WALSH, Minister for Agriculture, Food and Forestry, in exercise of the powers conferred on me by Section 3 of the European Communities Act, 1972 (No. 27 of 1972), and for the purposes of giving effect to Commission Directive 92/95/EEC of 9 November, 1992(1), hereby make the following Regulations: 1. (1) These Regulations may be cited as the European Communities (Feedingstuffs) (Methods of Analysis) (Amendment) Regulations, 1993. | |||||||||||||||||||||||||||||
(2) The European Communities (Feedingstuffs) (Methods of Analysis) Regulations 1978 to 1985, and these Regulations may be cited together as the European Communities (Feedingstuffs) (Methods of Analysis) Regulations, 1978 to 1993. 2. The European Communities (Feedingstuffs) (Methods of Analysis) Regulations, 1978 ( S.I. No. 250 of 1978 ) is hereby amended by the substitution of the following paragraph for paragraph 23 of Part II: | |||||||||||||||||||||||||||||
"23 DETERMINATION OF AFLATOXIN B1 | |||||||||||||||||||||||||||||
(A) ONE DIMENSIONAL THIN LAYER CHROMATOGRAPHIC METHOD | |||||||||||||||||||||||||||||
1. Purpose and Scope | |||||||||||||||||||||||||||||
To determine the level of aflatoxin B1 in raw materials and straight feedingstuffs. This method can not be applied in the presence of citrus pulp. In the presence of interfering substances it is necessary to repeat the analysis using method B (High Performance Liquid Chromatography). The lower limit of determination is 0.01 mg/kg (10 ppb). | |||||||||||||||||||||||||||||
2. Principle | |||||||||||||||||||||||||||||
The sample is subjected to extraction with chloroform. The extract is filtered, and an aliquot portion taken and purified by column chromatography on silica gel. The eluate is evaporated and the residue redissolved in a specific volume of chloroform or of a mixture of benzene and acetonitrile. An aliquot portion of this solution is subjected to thin-layer chromatography (TLC). The quantity of aflatoxin B1 is determined under UV irradiation of the chromatogram, either visually or by flourodensitometry, by comparison with known quantities of standard aflatoxin B1. The identity of the aflatoxin B1 extracted from the feedingstuff must be confirmed by the procedure indicated. | |||||||||||||||||||||||||||||
(1)O.J. No. L327 13-11-1992. p.54. | |||||||||||||||||||||||||||||
3. Reagents | |||||||||||||||||||||||||||||
3.1 Acetone. | |||||||||||||||||||||||||||||
3.2 Chloroform, stabilized with 0.5 — 1.0% of 96% ethanol (v/v). | |||||||||||||||||||||||||||||
3.3 N-hexane. | |||||||||||||||||||||||||||||
3.4 Methanol. | |||||||||||||||||||||||||||||
3.5 Anhydrous diethyl ether, free from peroxides. | |||||||||||||||||||||||||||||
3.6 Mixture of benzene and acetonitrile: 98/2 (v/v). | |||||||||||||||||||||||||||||
3.7 Mixture of chloroform (3.2) and methanol (3.4): 97/3 (v/v). | |||||||||||||||||||||||||||||
3.8 Silica gel, for column chromatography, particle size 0.05—0.20 mm. | |||||||||||||||||||||||||||||
3.9 Absorbent cotton wool, previously defatted with chloroform, or glass wool. | |||||||||||||||||||||||||||||
3.10 Sodium sulphate, anydrous, granular. | |||||||||||||||||||||||||||||
3.11 Inert gas, e.g. nitrogen. | |||||||||||||||||||||||||||||
3.12 Hydrochloric acid solution, 1 N. | |||||||||||||||||||||||||||||
3.13 Sulphuric acid solution, 50% (v/v). | |||||||||||||||||||||||||||||
3.14 Kieselguhr (hyflosupercel), washed in acid. | |||||||||||||||||||||||||||||
3.15 Silica gel G-HR or equivalent, for TLC. | |||||||||||||||||||||||||||||
3.16 Standard solution with about 0.1 µg of aflatoxin B1 per ml in chloroform (3.2) or the benzene/ acetonitrile mixture (3.6), prepared and checked as indicated in Section 7. | |||||||||||||||||||||||||||||
3.17 Standard solution for qualitative testing purposes containing about 0.1 µg of aflatoxin B1 and B2 per ml in chloroform (3.2) or the benzene/acetonitrile mixture (3.6). These concentrations are given as a guide. They must be adjusted so as to obtain the same intensity of fluorescence for both aflatoxins. | |||||||||||||||||||||||||||||
3.18 Developing solvents: | |||||||||||||||||||||||||||||
3.18.1 Chloroform (3.2)/acetone (3.1): 9/1 (v/v), unsaturated tank; | |||||||||||||||||||||||||||||
3.18.2 Diethyl ether (3.5)/methanol (3.4)/water: 96/3/1 (v/v/v), unsaturated tank; | |||||||||||||||||||||||||||||
3.18.3 Diethyl ether (3.5)/methanol (3.4)/water: 94/4.5/1.5 (v/v/v), saturated tank; | |||||||||||||||||||||||||||||
3.18.4 Chloroform (3.2)/methanol (3.4): 94/6 (v/v), saturated tank; | |||||||||||||||||||||||||||||
3.18.5 Chloroform (3.2)/methanol (3.4): 97/3 (v/v), saturated tank. | |||||||||||||||||||||||||||||
4. Apparatus | |||||||||||||||||||||||||||||
4.1 Grinder-mixer. | |||||||||||||||||||||||||||||
4.2 Shaking apparatus or magnetic stirrer. | |||||||||||||||||||||||||||||
4.3 Fluted filter papers, Schleicher and Schull No. 588 or equivalent, diameter: 24 cm. | |||||||||||||||||||||||||||||
4.4 Glass tube for chromatography (internal diameter: 22 mm approximately, length: 300 mm approximately), with a PTFE cock and a suitable reservoir. | |||||||||||||||||||||||||||||
4.5 Rotary vacuum evaporator, with a 500 ml round-bottom flask. | |||||||||||||||||||||||||||||
4.6 500 ml conical flasks, with ground-glass stoppers. | |||||||||||||||||||||||||||||
4.7 TLC apparatus. | |||||||||||||||||||||||||||||
4.8 Glass plates for TLC, 200 x 200 mm, prepared as follows (the quantities indicated are sufficient to cover five plates). Put 30 g of silica gel G-HR (3.15) into a conical flask. Add 60 ml water, stopper and shake for a minute. Spread the suspension on the plates so as to obtain a uniform layer 0.25 mm thick. Leave to dry in the air and then store in a desiccator containing silica gel. At the time of use, activate the plates by keeping them in the oven at 110°C for 1 hour. | |||||||||||||||||||||||||||||
Ready-to-use plates are suitable if they give results similar to those obtained with the plates prepared as indicated above. | |||||||||||||||||||||||||||||
4.9 Long-wavelength (360 nm) UV lamp. The intensity of irradiation must make it possible for a spot of 1 ng of aflatoxin B1 to be still clearly distinguished on a TLC plate at a distance of 10 cm from the lamp. | |||||||||||||||||||||||||||||
4.10 10 ml graduated tubes with polyethylene stoppers. | |||||||||||||||||||||||||||||
4.11 UV spectrophotometer. | |||||||||||||||||||||||||||||
4.12 Fluorodensitometer (optional). | |||||||||||||||||||||||||||||
5. Procedure | |||||||||||||||||||||||||||||
5.1 Preparation of the sample (see under "Observations", Point 10) | |||||||||||||||||||||||||||||
Grind the sample so that the whole of it will pass through a sieve with a 1 mm mesh (in accordance with recommendation ISO R 565). | |||||||||||||||||||||||||||||
5.2 Extraction | |||||||||||||||||||||||||||||
Put 50 g of ground, mixed sample into a conical flask (4.6). Add 25 g of Kieselguhr (3.14), 25 ml of water and 250 ml of chloroform (3.2). Stopper the flask, shake or stir for 30 minutes with the apparatus (4.2) and filter through a fluted filter paper (4.3). Discard the first 10 ml of the filtrate and then collect 50 ml. | |||||||||||||||||||||||||||||
5.3 Column clean up | |||||||||||||||||||||||||||||
Insert into the lower end of a chromatography tube (4.4) a cotton or glass wool plug (3.9), fill two-thirds of the tube with chloroform (3.2) and add 5 g of sodium sulphate (3.10). | |||||||||||||||||||||||||||||
Check that the upper surface of the sodium sulphate layer is flat, then add 10 g of silica gel (3.8) in small portions. Stir carefully after each addition to eliminate air bubbles. Leave to stand for 15 minutes and then carefully add 15 g of sodium sulphate (3.10). Let the liquid fall until it is just above the upper surface of the sodium sulphate layer. | |||||||||||||||||||||||||||||
Mix the 50 ml of extract collected in 5.2 with 100 ml of n-hexane (3.3) and quantitatively transfer the mixture to the column. Let the liquid fall until it is just above the upper surface of the sodium sulphate layer. Discard this washing. Then add 100 ml of diethylether (3.5) and again allow it to fall to the upper surface of the sodium sulphate layer. Discard this washing. Then add 100 ml of diethylether (3.5) and again allow it to fall to the upper surface of the sodium sulphate layer. During these operations see that the rate of flow is 8—12 ml per minute and that the column does not run dry. Discard the liquid that comes out. Then elute with 150 ml of the chloroform/methanol mixture (3.7) and collect the whole of the eluate. Evaporate the latter almost to dryness at a temperature not exceeding 50°C under a stream of inert gas (3.11) with the rotary evaporator (4.5). Quantitatively transfer the residue, using chloroform (3.2) or the benzeneacetonitrile mixture (3.6), to a 10 ml graduated tube (4.10). Concentrate the solution under a stream of inert gas (3.11) and then adjust the volume to 2 ml with chloroform (3.2) or the benzene/acetonitrile mixture (3.6). | |||||||||||||||||||||||||||||
5.4 Thin-layer chromatography | |||||||||||||||||||||||||||||
Spot on a TLC plate (4.8), 2 cm from the lower edge and at intervals of 2 cm, the volumes indicated below of the standard solution and the extract: | |||||||||||||||||||||||||||||
—10, 15, 20, 30 and 40 µl of the standard aflatoxin B1 solution (3.16); | |||||||||||||||||||||||||||||
—10 µl of the extract obtained in 5.3 and, superimposed on the same point, 20 µl of the standard solution (3.16); | |||||||||||||||||||||||||||||
—10 and 20 µl of the extract obtained in 5.3. | |||||||||||||||||||||||||||||
Develop the chromatogram in the dark with one of the developing solvents (3.18). The choice of the solvent must be made beforehand, by depositing 25 µl of the qualitative standard solution (3.17) on the plate and checking that, when developed, aflatoxin B1 and B2 are completely separated. | |||||||||||||||||||||||||||||
Let the solvents evaporate in the dark and then irradiate with UV light, placing the plate 10 cm from the lamp (4.9). The spots of aflatoxin B1 give a blue fluorescence. | |||||||||||||||||||||||||||||
5.5 Quantitative determinations | |||||||||||||||||||||||||||||
Determine either visually or by fluorodensitometry as indicated below. | |||||||||||||||||||||||||||||
5.5.1 Visual measurements | |||||||||||||||||||||||||||||
Determine the quantity of aflatoxin B1 in the extract by matching the fluorescence intensity of the extract spots with that of one of the standard solution spots. Interpolate if necessary. The fluorescence obtained by the superimposition of the extract on the standard solution must be more intense than that of the 10 µl of extract and there must not be more than one visible spot. If the fluorescence intensity given by the 10 µl of extract is greater than that of the 40 µl of standard solution, dilute the extract 10 or 100 times with chloroform (3.2) or the benzene/acetonitrile mixture (3.6) before subjecting it again to thin-layer chromatography. | |||||||||||||||||||||||||||||
5.5.2 Measurements by fluorodensitometry | |||||||||||||||||||||||||||||
Measure the fluorescence intensity of the aflatoxin B1 spots with the fluorodensitometer (4.12) at an excitation wavelength of 365 nm and an emission wavelength of 443 nm. Determine the quantity of aflatoxin B1 in the extract spots by comparison of their fluorescence intensities with that of the standard aflatoxin B1 spots. | |||||||||||||||||||||||||||||
5.6 Confirmation of the identity of aflatoxin B1 | |||||||||||||||||||||||||||||
Confirm the identity of the aflatoxin B1 in the extract by the processes indicated below. | |||||||||||||||||||||||||||||
5.6.1 Treatment with sulphuric acid | |||||||||||||||||||||||||||||
Spray sulphuric acid (3.13) on to the chromatogram obtained in 5.4. The fluorescence of the aflatoxin B1 spots must turn from blue to yellow under UV irradiation. | |||||||||||||||||||||||||||||
5.6.2 Two-dimensional chromatography involving the formation of aflatoxin B1-hemiacetal (aflatoxin B2a) | |||||||||||||||||||||||||||||
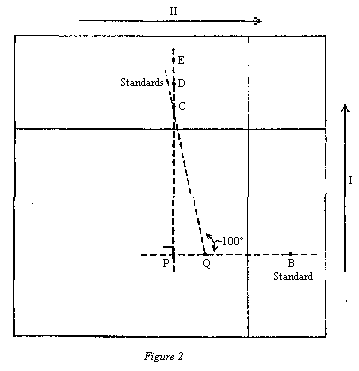
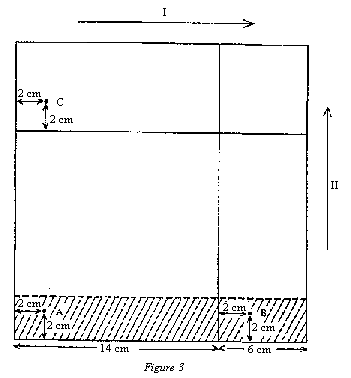
NB: The operations described below must be carried out following carefully the diagram in figure 3. | |||||||||||||||||||||||||||||
5.6.2.1 Application of the solutions | |||||||||||||||||||||||||||||
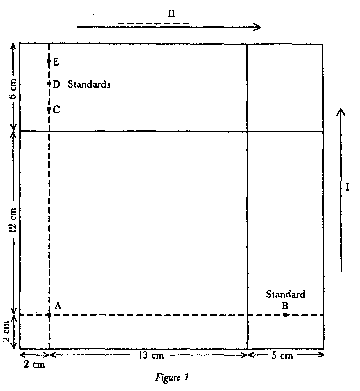
Score two straight lines on the plate (4.8) parallel to two contiguous sides (6 cm in from each side) to limit migration of the solvent fronts. Spot the following solutions on the plate using capillary pipettes or microsyringes: | |||||||||||||||||||||||||||||
— on point A: a volume of purified extract of sample, obtained in 5.3, containing about 2.5 nm of aflatoxin B1; | |||||||||||||||||||||||||||||
— on points B and C: 25 µl of the standard solution (3.16). | |||||||||||||||||||||||||||||
5.6.2.2 Development | |||||||||||||||||||||||||||||
Develop the chromatogram in direction I, in the dark, using the developing solvent (3.18.1) (1 cm layer in an unsaturated tank) until the solvent front reaches the solvent limit line. Remove the plate from the tank and allow to dry in the dark at ambient temperature for five minutes. Then spray hydrochloric acid (3.12) along a band 2.5 cm high, covering points A and B (indicated by the hatched area in figure 3) until it darkens, protecting the rest of the plate with a glass sheet. Allow to react for 10 minutes in the dark and dry with a stream of air at ambient temperature. | |||||||||||||||||||||||||||||
Next, develop the chromatogram in direction II, in the dark, using the developing solvent (3.18.1) (1 cm layer in an unsaturated tank) until the solvent front reaches the solvent limit line. Remove the plate from the tank and allow to dry at ambient temperature. | |||||||||||||||||||||||||||||
5.6.2.3 Interpretation of the chromatogram | |||||||||||||||||||||||||||||
Examine the chromatogram under UV light (4.9) and check for the following features. | |||||||||||||||||||||||||||||
( a ) Appearance of a blue fluorescent spot of aflatoxin B1 originating from the standard solution applied at C (migration in direction I). | |||||||||||||||||||||||||||||
( b ) Appearance of a blue fluorescent spot of unreacted (with the hydrochloric acid) aflatoxin B1 and a more intense blue fluorescent spot of aflatoxin B1-hemiacetal, both originating from the standard solution applied at B (migration in direction II). | |||||||||||||||||||||||||||||
( c ) Appearance of spots matching those described in (b), originating from the sample extract applied at A. The position of these spots is defined first by the migration distance of the aflatoxin B1 from point A in direction I (the same as that travelled by the standard applied at C), and then by the migration distances from there in direction II of the aflatoxin B1-hemiacetal (same as those travelled by the standard applied at B). The fluorescence intensities of hemiacetal spots originating from the extract and from the standard applied at B should match. | |||||||||||||||||||||||||||||
6. Calculation of results | |||||||||||||||||||||||||||||
6.1 From the visual measurements | |||||||||||||||||||||||||||||
The content in micrograms of aflatoxin B1 per kg of sample (ppb) is given by the formula: | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
in which: | |||||||||||||||||||||||||||||
Y and Z are respectively the volumes in microlitres of the standard solution of aflatoxin B1 (3.16) and of the extract having similar intensity of fluorescence; | |||||||||||||||||||||||||||||
S = concentration in micrograms of aflatoxin B1 per ml in the standard solution (3.16); | |||||||||||||||||||||||||||||
V = final volume of the extract in microlitres, allowing for any dilution that was necessary; | |||||||||||||||||||||||||||||
W = weight in grams of the sample corresponding to the volume of extract subjected to column clean-up. | |||||||||||||||||||||||||||||
6.2 From the fluorodensitometric measurements | |||||||||||||||||||||||||||||
The content in micrograms of aflatoxin B1 per kg of sample is given by the formula: | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
in which: | |||||||||||||||||||||||||||||
Y = volume in microlitres of the extract spotted on the plate (10 or 20 µl); | |||||||||||||||||||||||||||||
S = quantity in nanograms of aflatoxin B1 in the extract spot (proportional to the value of Y taken), deducted from the measurements; | |||||||||||||||||||||||||||||
V = final volume of the extract in microlitres, allowing for any dilution that was necessary; | |||||||||||||||||||||||||||||
W = weight in grams of the sample corresponding to the volume of extract subjected to column clean-up. | |||||||||||||||||||||||||||||
7. Preparation and testing of the standard solution (3.16) | |||||||||||||||||||||||||||||
7.1 Determination of the concentration of aflatoxin B1 | |||||||||||||||||||||||||||||
Prepare a standard solution of aflatoxin B1 in the chloroform (3.2) or the benzene/acetonitrile mixture (3.6) with a concentration of 8 to 10 µg/ml. Determine the absorption spectrum between 330—370 nm with the aid of the spectrophotometer (4.11). | |||||||||||||||||||||||||||||
Measure the optical density (A) at 363 nm in the case of the chloroform solution; or at 348 nm in the case of the solution in the benzene/acetonitrile mixture. | |||||||||||||||||||||||||||||
Calculate the concentration in micrograms of aflatoxin B1 per ml of solution from the formulae below: | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
Dilute as appropriate, away from day light, to obtain a working standard solution with a concentration of aflatoxin B1 of about 0.1 µg/ml. If kept in a refrigerator at 4°C , this solution is stable for two weeks. | |||||||||||||||||||||||||||||
7.2 Testing of chromatographic purity | |||||||||||||||||||||||||||||
Spot on a plate (4.8) 5 µl of the standard solution of aflatoxin B1 containing 8 to 10 µg/ml (7.1). Develop the chromatogram as indicated in 5.4. In UV light the chromatogram should show only one spot and no fluorescence must be perceptible in the original deposit zone. | |||||||||||||||||||||||||||||
8. Repeatability | |||||||||||||||||||||||||||||
The difference between the results of two parallel determinations carried out on the same sample by the same analyst should not exceed: | |||||||||||||||||||||||||||||
— 25% relative to the highest result, for contents of aflatoxin B1 from 10 and up to 20 µg/kg; | |||||||||||||||||||||||||||||
— 5 µg, in absolute value, for contents greater than 20 and up to 50 µg/kg; | |||||||||||||||||||||||||||||
— 10% relative to the highest value, for contents above 50 µg/kg. | |||||||||||||||||||||||||||||
9. Reproducibility | |||||||||||||||||||||||||||||
The Reproducibility of the results, i.e. the variation between the results obtained by two or more laboratories on the same sample has been estimated at: | |||||||||||||||||||||||||||||
± 50% of the mean value, for mean values of aflatoxin B1 from 10 up to 20 µg/kg; | |||||||||||||||||||||||||||||
± 10 µg/kg on the mean value, for mean values of greater than 20 and up to 50 µg/kg; | |||||||||||||||||||||||||||||
± 20% of the mean value, for mean values above 50 µg/kg. | |||||||||||||||||||||||||||||
10. Observations | |||||||||||||||||||||||||||||
Defatting | |||||||||||||||||||||||||||||
Samples containing more than 5% fats must be defatted with light petroleum (bp 40 to 60°C) after the preparation indicated in 5.1. In such cases, the analytical results must be expressed in terms of the weight of the non-defatted sample. | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
Figure 2 : Flow diagram of the LC system with iodine post-column derivatization | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
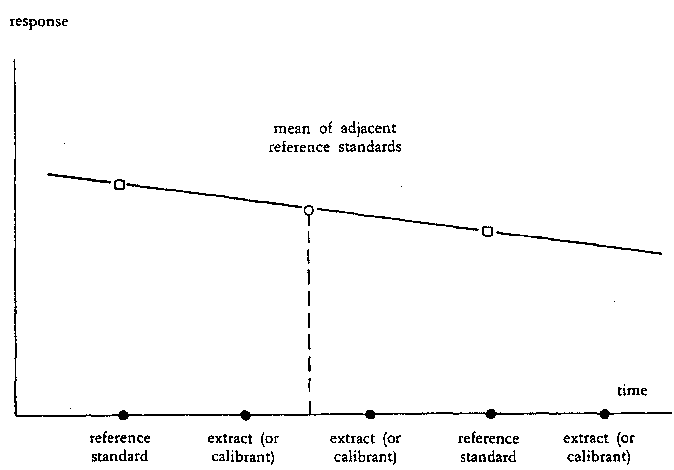
Figure 3 : Compensation for drift in aflatoxin B1 respond by injecting reference standard (3.13.3) at regular intervals' | |||||||||||||||||||||||||||||
(B) HIGH PERFORMANCE LIQUID CHROMATOGRAPHIC METHOD | |||||||||||||||||||||||||||||
1. Purpose and Scope | |||||||||||||||||||||||||||||
To determine the level of aflatoxin B1 in animal feedingstuffs including those containing citrus pulp. The lower limit of determination is 0.001 mg/kg (1 ppb). | |||||||||||||||||||||||||||||
2. Principle | |||||||||||||||||||||||||||||
The sample is extracted with chloroform. The extract is filtered and an aliquot portion is purified by elution through a Florisil cartridge followed by elution through a C18 cartridge. The purified extract is subjected to reversed phase HPLC, followed by post-column derivatization with iodine in water, and fluorescence detection of the aflatoxin B1 derivative. | |||||||||||||||||||||||||||||
3. Reagents | |||||||||||||||||||||||||||||
Warning: | |||||||||||||||||||||||||||||
Mycotoxins are extremely toxic substances. Manipulations should be performed in a designated fume cupboard. Special precautions should be taken when toxins are in a dry form because of their electrostatic nature and resulting tendency to disperse in working areas. | |||||||||||||||||||||||||||||
Caution: | |||||||||||||||||||||||||||||
— Protect the laboratory, where the analyses are done, adequately from daylight. | |||||||||||||||||||||||||||||
This can be achieved effectively by using: | |||||||||||||||||||||||||||||
( i ) UV absorbing foil on the windows in combination with subdued light (no direct sunlight); | |||||||||||||||||||||||||||||
( ii ) Curtains or blinds in combination with artificial light (fluorescent tubes are acceptable); | |||||||||||||||||||||||||||||
— Aflatoxin containing solutions must be protected from light as much as possible (keep in the dark, use aluminium foil). | |||||||||||||||||||||||||||||
— Use of non acid-washed glassware for aqueous aflatoxin solutions may cause losses. Particular care should be taken with new glassware and disposable glassware such as autosampler vials and Pasteur pipettes. Therefore laboratory glassware coming into contact with aqueous solutions of aflatoxins should be soaked in dilute acid (e.g. sulphuric acid = 2 mol/l) for several hours, then rinsed well with distilled water to remove all traces of acid (e.g. three times, check with pH-paper). In practice, this treatment is necessary for the round bottomed flask (4.2), the volumetric flasks, measuring cylinders, vials or tubes used for calibration solutions and final extracts (particularly vials for autosamplers), and Pasteur pipettes, if these are used to transfer calibration solutions or extracts. | |||||||||||||||||||||||||||||
3.1 Chloroform, stabilized with 0.5 to 1.0% of ethanol, by mass. See observation 10.2. | |||||||||||||||||||||||||||||
3.2 Methanol, HPLC grade. | |||||||||||||||||||||||||||||
3.3 Acetone. | |||||||||||||||||||||||||||||
3.4 Acetonitrile, HPLC grade. | |||||||||||||||||||||||||||||
3.5 Eluting solvents: Prepare one day before use, or remove air in the solvents ultrasonically. | |||||||||||||||||||||||||||||
3.5.1 Mixture of acetone (3.3) and water, 98 + 2 (v + v). | |||||||||||||||||||||||||||||
3.5.2 Mixture of water and methanol (3.2), 80 + 20 (v + v). | |||||||||||||||||||||||||||||
3.5.3 Mixture of water and acetone (3.3) 85 + 15 (v + v). | |||||||||||||||||||||||||||||
3.6 Mobile phase for HPLC | |||||||||||||||||||||||||||||
Mixture of water, methanol (3.2) and acetonitrile (3.4), 130 + 70 + 40 (v + v + v). | |||||||||||||||||||||||||||||
NB The composition of the Mobile phase solvent may need to be adjusted, depending on the characteristics of the HPLC column used. | |||||||||||||||||||||||||||||
3.7 Saturated iodine solution: add 2 g of iodine to 400 ml of water. Mix for at least 90 minutes and filter through a membrane filter (4.13). Protect the saturated solution from light to prevent photodegradation. | |||||||||||||||||||||||||||||
3.8 Acid washed Celite 545, or equivalent. | |||||||||||||||||||||||||||||
3.9 Florisil cartridge (Waters SEP-PAK), or equivalent. | |||||||||||||||||||||||||||||
3.10 C18 cartridge (Waters SEP-PAK), or equivalent. | |||||||||||||||||||||||||||||
3.11 Inert gas e.g. nitrogen. | |||||||||||||||||||||||||||||
3.12 Aflatoxin B1 standard solution in chloroform, concentration 10 µg/ml. | |||||||||||||||||||||||||||||
Check the concentration of the solution as follows: determine the absorption spectrum of the solution between 330 and 370 nm by means of the spectrophotometer (4.21). Measure the absorbency (A) at the maximum near 363 nm. Calculate the concentration of aflatoxin B1 in micrograms per millilitre of solution from the formula. | |||||||||||||||||||||||||||||
Concentration (µg/ml) = | |||||||||||||||||||||||||||||
3.12.1 Aflatoxin B1 stock standard solution in chloroform. | |||||||||||||||||||||||||||||
Transfer quantitatively 2.5 ml of the aflatoxin B1 standard solution (3.12) to a 50 ml volumetric flask and adjust to the mark with chloroform (3.1). Store this solution in a cool place (4°C) in the dark, well sealed and wrapped in aluminium foil. | |||||||||||||||||||||||||||||
3.13 Aflatoxin B1 calibration solutions HPLC. | |||||||||||||||||||||||||||||
NB: Use acid-washed glassware for preparation of these solutions (see Caution, third indent). | |||||||||||||||||||||||||||||
3.13.1 Calibration solution 4 ng/ml. | |||||||||||||||||||||||||||||
Allow the volumetric flask with stock standard solution (3.12.1) to warm up to room temperature in the aluminium foil (a few hours). Transfer 400 µl of stock standard solution (200 ng aflatoxin B1) into a 50 ml volumetric flask, and evaporate the solution to dryness in a current of inert gas (3.11). Dissolve the residue obtained in approximately 20 ml of water/acetone mixture (3.5.3), make up to the mark with the water/acetone mixture and mix well. | |||||||||||||||||||||||||||||
3.13.2 Calibration solution 3 ng/ml. | |||||||||||||||||||||||||||||
Transfer quantitatively 7.5 ml of the calibration solution (3.13.1) into a 10 ml volumetric flask, make up to the mark with the water/acetone mixture (3.5.3), and mix well. | |||||||||||||||||||||||||||||
3.13.3 Calibration solution, 2 ng/ml. | |||||||||||||||||||||||||||||
Transfer quantitatively 25 ml of the calibration solution (3.13.1) to a 50 ml volumetric flask, make up to the mark with the water/acetone mixture (3.5.3) and mix well. | |||||||||||||||||||||||||||||
This solution is also referred to as the reference standard, to be used for repetitive injection during HPLC (5.3). | |||||||||||||||||||||||||||||
3.13.4 Calibration solution 1 ng/ml. | |||||||||||||||||||||||||||||
Transfer quantitatively 2.5 ml of the calibration solution (3.13.1) to a 10 ml volumetric flask, make up to the mark with the water/acetone mixture (3.5.3) and mix well. | |||||||||||||||||||||||||||||
3.14 Ampoule containing a mixture of aflatoxins B1, B2, G1 and G2 concentrations approximately 1, 0.5, 1 and 0.5 µg/ml respectively, in 1 ml chloroform. | |||||||||||||||||||||||||||||
3.14.1 Chromatographic test solution | |||||||||||||||||||||||||||||
Transfer the content of the ampoule (3.14) into a glass-stoppered test-tube or screw-capped vial. Transfer 40 µl of this solution into a glass-stoppered test-tube (acid rinsed) (4.20). Evaporate the chloroform in a stream of inert gas (3.11) and redissolve into 10 ml of the water/acetone mixture (3.5.3). | |||||||||||||||||||||||||||||
3.15 Reagents for confirmatory test (6). | |||||||||||||||||||||||||||||
3.15.1 Sodium chloride saturated solution. | |||||||||||||||||||||||||||||
3.15.2 Sodium sulphate, anhydrous, granular. | |||||||||||||||||||||||||||||
4. Apparatus | |||||||||||||||||||||||||||||
4.1 Mechanical shaker. | |||||||||||||||||||||||||||||
4.2 Rotary vacuum evaporator, equipped with a 150 to 250 ml round bottomed flask. | |||||||||||||||||||||||||||||
4.3 High performance liquid chromatograph, containing an injector with a loop suitable for the injection of 250 ml. See the manufacturers instructions for partial or complete loop filling. | |||||||||||||||||||||||||||||
4.4 HPLC analytical column: 3 µm or 5 µm C18 packing. | |||||||||||||||||||||||||||||
4.5 Pulse-free pump for delivery of the iodine postcolumn reagent. | |||||||||||||||||||||||||||||
4.6 Valco zero dead volume Tee, stainless steel (1/16" x 0.75 mm). | |||||||||||||||||||||||||||||
4.7 Spiral reaction coil; Teflon or stainless steel. Dimensions of 3000 X0.5 mm to 5000 x 0.5 mm have been found to be appropriate in combination with 5 µm or 3 µm HPLC columns. | |||||||||||||||||||||||||||||
4.8 Thermostatically controlled water-bath adjusted to 60°C capable of temperature regulation to better than 0.1°C. | |||||||||||||||||||||||||||||
4.9 Fluorescence detector, with excitation at 365 nm and emission at 435 nm wavelengths. (For filter instrument: emission wavelength > 400 nm). Detection of at least 0.05 ng aflatoxin B1 shall be possible. Some back pressure may be advisable (e.g. restrictor, Teflon or stainless steel coil connected to the outlet of the detector), to suppress air bubbles in the flow-cell. | |||||||||||||||||||||||||||||
4.10 Strip chart recorder. | |||||||||||||||||||||||||||||
4.11 Electronic integrator (optional). | |||||||||||||||||||||||||||||
4.12 Fluted filter paper, diameter: 24 cm, Macherey-Nagel 617 ¼ or equivalent. | |||||||||||||||||||||||||||||
4.13 Membrane filter with a pore size of 0.45 µm. Millipore HAWP 04700 or equivalent. | |||||||||||||||||||||||||||||
4.14 500 ml glass stoppered conical flask. | |||||||||||||||||||||||||||||
4.15 Glass column (internal diameter approximately 1 cm, length approximately 30 cm) equipped with a Luer tip. | |||||||||||||||||||||||||||||
4.16 Luer chloroform-resistant stopcock (e.g. Bio-rad 7328017, Analytichem A1 6078, J. T. Baker 4514 or equivalent). | |||||||||||||||||||||||||||||
4.17 Chemically resistant syringe, 10 ml Luer connector. | |||||||||||||||||||||||||||||
4.18 Syringe suitable for HPLC injection of 250 µl (see 4.3). | |||||||||||||||||||||||||||||
4.19 100 µl microsyringe for preparation of calibration solutions (check that the accuracy is within 2% by weighing). | |||||||||||||||||||||||||||||
4.20 10 ml glass stoppered calibrated tubes. | |||||||||||||||||||||||||||||
4.21 Spectrophotometer, suitable for making measurements in the UV region of the spectrum. | |||||||||||||||||||||||||||||
4.22 Equipment for confirmatory test (6). | |||||||||||||||||||||||||||||
4.22.1 Acid-rinsed 100 ml separating funnel with Teflon stopcock. | |||||||||||||||||||||||||||||
4.22.2 Heating block, 40 to 50°C. | |||||||||||||||||||||||||||||
5. Procedure | |||||||||||||||||||||||||||||
5.1 Extraction | |||||||||||||||||||||||||||||
Weigh 50 g of the prepared sample (see Observations 10.4) into the conical flask (4.14) and add 25 g of Celite (3.8), 250 ml of chloroform (3.1) and 25 ml of water. Stopper the flask, and shake for 30 minutes on a mechanical shaker (4.1). Filter through a fluted filter paper (4.12). Collect 50 ml of the filtrate. If necessary, take an aliquot of the filtrate and dilute to 50 ml with chloroform so that the concentration of aflatoxin B1 is not greater than 4 ng/ml. | |||||||||||||||||||||||||||||
5.2 Clean-up (the procedure should be carried out without significant interruptions). | |||||||||||||||||||||||||||||
5.2.1 Florisil SEP-PAK purification. | |||||||||||||||||||||||||||||
5.2.1.1 Preparation of the column-cartridge assembly. | |||||||||||||||||||||||||||||
Attach a stopcock (4.16) to the shorter stem of a Florisil cartridge (3.9) (see figure 1). Wash the cartridge and remove air by taking 10 ml chloroform (3.1) and passing 8 ml via the stopcock rapidly through the cartridge using a syringe (4.17). | |||||||||||||||||||||||||||||
Attach the longer stem of the cartridge to a glass column (4.15) and pass the remaining 2 ml chloroform through the cartridge into the column. Close the stopcock. Remove the syringe. | |||||||||||||||||||||||||||||
5.2.1.2 Purification | |||||||||||||||||||||||||||||
Add the filtrate collected in 5.1 to the column-cartridge assembly and drain by gravity. Rinse with 5 ml of chloroform (3.1), followed by 20 ml of methanol (3.2). Discard the elutes. Ensure that the column-cartridge assembly does not run dry. | |||||||||||||||||||||||||||||
Elute aflatoxin B1 with 40 ml of the acetone-water mixture (3.5.1) and collect all of the eluate in the round bottomed flask of the rotary evaporator (4.2). Concentrate the eluate on the rotary evaporator at 40°C too 50°C until all the acetone is distilled. (NB approximately 0.5 ml of liquid remains in the flask at this point. Experiments have shown that further evaporation is not harmful and that when 0.5 ml of liquid remains, there is then no significant amount of acetone. Residues of acetone might lead to losses of aflatoxin B1 on the C18 cartridge). Add 1 ml of methanol (3.2), swirl the flask to dissolve aflatoxin B1 on the sides of the flask, add 4 ml water, and mix. Disconnect and discard the cartridge. Rinse the glass column with water and retain for the C18 purification step. | |||||||||||||||||||||||||||||
5.2.2 C18 SEP-PAK Purification. | |||||||||||||||||||||||||||||
5.2.2.1 Preparation of the column-cartridge assembly. | |||||||||||||||||||||||||||||
Attach a stopcock (4.16) to the shorter stem of a C18 cartridge (3.10) (see figure 1). Prime the cartridge and remove any air by passing 10 ml of methanol (3,2) via the stopcock rapidly through the cartridge with a syringe (4.17). (Air bubbles in the cartridge are visible as light spots on the otherwise greyish background). Take 10 ml water, and pass 8 ml through the cartridge. (Avoid introduction of air into the cartridge, when switching from methanol to water). Attach the longer stem of the cartridge to a glass column (4.15) and pass the remaining 2 ml water through the cartridge in the column. Close the stopcock. Remove the syringe. | |||||||||||||||||||||||||||||
5.2.2.2 Purification | |||||||||||||||||||||||||||||
Transfer the extract collected in the 5.2.1.2 quantitatively to the glass column (4.15) and rinse the flask twice with 5 ml water/methanol mixture (3.5.2) and drain by gravity. During these operations, ensure that the column-cartridge assembly does not run dry. (If air bubbles develop in the constriction near the cartridge, stop the flow and tap the top of the glass column, to remove them). Elute with 25 ml water/methanol mixture. Discard the eluate. Elute the aflatoxin B1 with 50 ml water/acetone mixture (3.5.3), and collect all of the eluate in a 50 ml volumetric flask. Make up to the mark with water and mix. Proceed to paragraph 5.3.8. | |||||||||||||||||||||||||||||
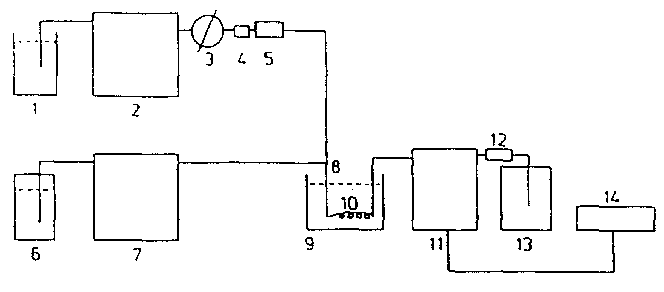
5.3 High performance liquid chromatography | |||||||||||||||||||||||||||||
(See figure 2 for assembly of the equipment). Allow sufficient time for conditioning and stabilizing the instruments. | |||||||||||||||||||||||||||||
Note 1: The flow-rates given for the Mobile phase and the post-column reagent are indicative only. They may need to be adjusted depending on the characteristics of the HPLC column. | |||||||||||||||||||||||||||||
Note 2: The detector response to aflatoxin B1 depends on the temperature, therefore compensation should be made for drift (see Figure 3). By injecting a fixed amount of aflatoxin B1 reference standard (3.13.3) at regular intervals (i.e. every third injection) the aflatoxin B1 peak values between these reference standards can be corrected using the mean response, provided that the difference between responses of consecutive reference standards is very small (< 10%). Injections must be made without interruptions. If interruption is necessary, the last injection before interruption and the first injection after interruption must be the reference standard (3.13.3). Because the calibration curve is linear and passes through the origin, the amounts of aflatoxin B1 in the sample extracts are determined directly by reference to the adjacent standards. | |||||||||||||||||||||||||||||
5.3.1 HPLC pump settings. | |||||||||||||||||||||||||||||
Using mobile phase (3.6), set the HPLC pump (4.3) to give a flow of 0.5 or 0.3 ml/min for a 5 µm or a 3 µm HPLC column (4.4), respectively. | |||||||||||||||||||||||||||||
5.3.2 Post-column pump settings. | |||||||||||||||||||||||||||||
Set the pump (4.5) to give a flow 0.2 to 0.4 ml/min of the iodine-saturated water solution (3.7). | |||||||||||||||||||||||||||||
As a rough guide: Flows of approximately 0.4 or 0.2 ml/min are advisable in combination with flows of 0.5 and 0.3 ml/min of the mobile phase (3.6) respectively. | |||||||||||||||||||||||||||||
5.3.3 Fluorescence detector. | |||||||||||||||||||||||||||||
Set the fluorescence detector (4.9) to exc. = 365 nm and em = 435 nm (filter instrument; > 400 nm). Adjust the detector attenuator to obtain approximately 80% full scale deflection of the recorder pen for 1 ng of aflatoxin B1. | |||||||||||||||||||||||||||||
5.3.4 Injector. | |||||||||||||||||||||||||||||
For all solutions, inject 250 µl amounts following the instructions of the manufacturer of the injector. | |||||||||||||||||||||||||||||
5.3.5 Check on chromatographic separation. | |||||||||||||||||||||||||||||
Inject the chromatographic test solution (3.14.1). Valleys should be less than 5% of the sum of peak heights of the adjacent peaks. | |||||||||||||||||||||||||||||
5.3.6 Check on the stability of the system. | |||||||||||||||||||||||||||||
Before each series of analyses, inject the reference standard (3.13.3), until stable peak areas are achieved. (NB: Peak responses for aflatoxin B1 between consecutive injections should not differ by more than 6%). Proceed without delay with the check of linearity (5.3.7). | |||||||||||||||||||||||||||||
5.3.7 Check for linearity. | |||||||||||||||||||||||||||||
Inject the aflatoxin B1 calibration solutions (3.13.1 to 3.13.4). Every third injection use the reference standard (3.13.3), for correction of drift in response. (NB: Peak responses for this references standard must not differ by more than 10% in 90 minutes). Correct the drift according to the formula in 7. The calibration graph should be linear and pass through the origin, within twice standard error of Y-estimate. Values found must not differ by more than 3% from the nominal values. If these requirements are fulfilled, continue without delay. If not, identify and correct the sources of the problem before proceeding to 5.3.8. | |||||||||||||||||||||||||||||
5.3.8 Chromatography of the sample extract (5.2.2.2). | |||||||||||||||||||||||||||||
Caution: It is normally not necessary to filter the final extract prior to HPLC. If it is considered necessary, cellulose filters must not be used, because they may lead to losses of aflatoxin B1. Teflon filters are acceptable. | |||||||||||||||||||||||||||||
Inject the purified sample extracts, obtained in paragraph 5.2.2.2, in the following sequence: reference standard (3.13.3), extract, extract, reference standard, extract, extract, reference standard, etc. | |||||||||||||||||||||||||||||
6. Confirmatory test | |||||||||||||||||||||||||||||
6.1 Further treatment of the extract (5.2.2.2). | |||||||||||||||||||||||||||||
Place the extract obtained in (5.2.2.2) into a separating funnel (4.22.1) and add 5 ml sodium chloride solution (3.15.1), and 2 ml chloroform (3.1). Shake for 2 minutes and repeat the extraction with 2 further 2 ml portions of chloroform (3.1). Pour the combined chloroform extracts over approximately 1 g sodium sulphate (3.15.2) into a 10 ml test tube. (A small funnel (diameter: 4 cm) can be used with a piece of cottonwool in the constriction, covered with approximately 1 g sodium sulphate). | |||||||||||||||||||||||||||||
Wash the sodium sulphate layer with a few ml of chloroform and combine with the chloroform extract. Evaporate the chloroform extract to dryness using a heating block (4.22.2). Dissolve the residue in 1 ml of chloroform and proceed to paragraph 5.6.2 of method A. | |||||||||||||||||||||||||||||
6.2 Preparation of derivative and thin layer chromatography. See Method A, point 5.6.2, Two-dimensional chromatography involving the formation of aflatoxin B1-hemiacetal (aflatoxin B2a). 7. Calculation of results | |||||||||||||||||||||||||||||
Calculate the aflatoxin B1 content (µg/kg) present in the sample, using the formula: | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
where: | |||||||||||||||||||||||||||||
m = amount of aflatoxin B1 in ng represented by the B1 peak of the sample, calculated as follows: | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
If the procedure is followed as in this protocol, the formula reduces to: aflatoxin B1 content in µg/kg = 20 x m. | |||||||||||||||||||||||||||||
7.1 Calculation of the results may also be done by peak height measurement. | |||||||||||||||||||||||||||||
8. Repeatability | |||||||||||||||||||||||||||||
See under 10.1. | |||||||||||||||||||||||||||||
9. Reproducibility | |||||||||||||||||||||||||||||
See under 10.1. | |||||||||||||||||||||||||||||
10. Observations | |||||||||||||||||||||||||||||
10.1 Precision | |||||||||||||||||||||||||||||
A collaborative study(1), carried out at international level on mixed feeding stuffs gave the results for repeatability and reproducibility indicated in Table 1. The term repeatability (r) used here is defined as the largest ratio which is not significant at the 95% probability level for comparison of two readings of the same sample in the same laboratory under similar conditions. The term reproducibility (R) is similarly defined for comparing two different laboratories. | |||||||||||||||||||||||||||||
In accordance to ISO 3534—1977, 2.35(2) and Commission Decision 89/610/EEC(3), r and R are also given in Table 1 in terms of coefficients of variation. | |||||||||||||||||||||||||||||
Table 1 | |||||||||||||||||||||||||||||
Repeatability (r) and reproducibility (R) expressed as ratios and corresponding coefficients of variation | |||||||||||||||||||||||||||||
(15 laboratories) | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
10.2. Stabilisation of chloroform | |||||||||||||||||||||||||||||
The absorption characteristics of the florisil cartridge may be changed if stabilizers other than ethanol are used. This should be verified in accordance with 10.3 when the chloroform described is not available. | |||||||||||||||||||||||||||||
(1)Egmond, H.P. van, Heisterkamp, S.H. and Paulsch, W.E. (1991). | |||||||||||||||||||||||||||||
(2)ISO 3534-1977. | |||||||||||||||||||||||||||||
(3)OJ No. L 351, 2-12-1989, p. 39. | |||||||||||||||||||||||||||||
(4)CV = Coefficient of Variation. | |||||||||||||||||||||||||||||
10.3 Accuracy | |||||||||||||||||||||||||||||
The correct applications of the method should be verified by making replicate measurements on certified reference materials. If these are not available, the performance of the method should be verified by recovery experiments made on the fortified blank samples. The deviation of the mean from the actual value, expressed as a percentage of the actual value, shall not lie outside the limits minus 20% to plus 10%. | |||||||||||||||||||||||||||||
10.4 Defatting | |||||||||||||||||||||||||||||
Samples containing more than 5% fats must be defatted with light petroleum (bp 40 to 60°C) after the preparation indicated in 5.1. | |||||||||||||||||||||||||||||
In such cases, the analytical result must be expressed in terms of the weight of the non-defatted sample. | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
GIVEN under my Official Seal, this 13th day of December, 1993. | |||||||||||||||||||||||||||||
JOE WALSH, | |||||||||||||||||||||||||||||
Minister for Agriculture, Food | |||||||||||||||||||||||||||||
and Forestry. | |||||||||||||||||||||||||||||
EXPLANATORY NOTE. | |||||||||||||||||||||||||||||
These Regulations amend the European Communities (Feedingstuffs) (Methods of Analysis) Regulations, 1978, (S.I. 250 of 1978) so as to give effect to Commission Directive 92/95/EEC which replaced the two-dimensional TLC method of analysis for aflatoxin B1 in feedingstuffs by a HPLC method. |

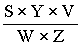
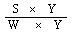
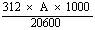 for the chloroform solution
for the chloroform solution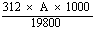 for the solution in the benzene/acetonitrile mixture
for the solution in the benzene/acetonitrile mixture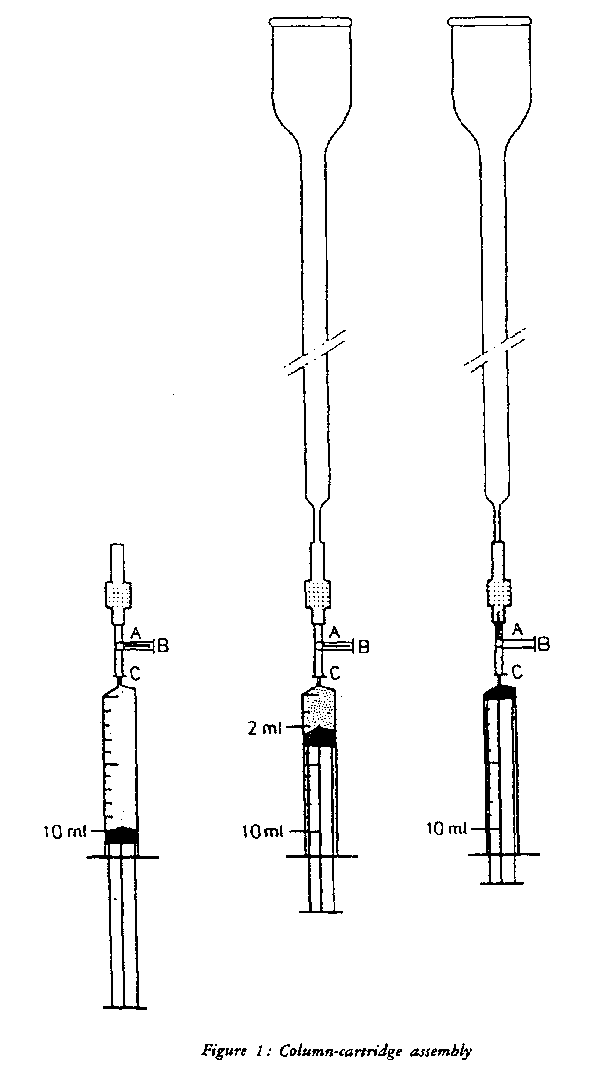
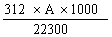 = 13.991 x A
= 13.991 x A