S.I. No. 77/1994 - European Communities (Classification, Packaging, Labelling and Notification of Dangerous Substances) Regulations, 1994.
ARRANGEMENT OF REGULATIONS. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
REGULATION. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SCHEDULE 1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Obligatory Headings for Safety Data Sheets. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SCHEDULE 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Risk Phrases. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table A—single Risk Phrases. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table B—combined Risk Phrases. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SCHEDULE 3. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Safety Phrases. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table A—single Safety Phrases. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table B—combined Safety Phrases. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SCHEDULE 4. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Danger Symbols and Indications of Danger. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SCHEDULE 5. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fees Payable by Notifier. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
S.I. No. 77 of 1994. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EUROPEAN COMMUNITIES (CLASSIFICATION, PACKAGING, LABELLING AND NOTIFICATION OF DANGEROUS SUBSTANCES) REGULATIONS, 1994. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
I, RUAIRÍ QUINN, Minister for Enterprise and Employment, in exercise of the powers conferred on me by section 3 of the European Communities Act, 1972 (No. 27 of 1972), and for the purpose of giving effect to Council Directive 92/32/EEC of 30 April 1992(1), and Commission Directives 92/69/EEC of 31 July, 1992(2), 93/21/EEC of 27 April, 1993(3) 93/67/EEC of 20 July, 1993(4), 93/72/EEC of 1 September, 1993(5), 93/90/EEC of 29 October, 1993(6), 93/101/EC of 11 November, 1993(7), 93/105/EC of 25 November, 1993(8), and 93/112/EC of 10 December, 1993(9), hereby make the following Regulations: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1 Short title | 1. These Regulations may be cited as the European Communities (Classification, Packaging, Labelling and Notification of Dangerous Substances) Regulations, 1994. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2 Interpretation | 2. (1) In these Regulations— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex I" means Annex I (inserted by Commission Directives 93/72/EEC of 1 September, 1993, and 93/101/EC of 11 November, 1993) to Council Directive 67/548/EEC (10); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex II" means Annex II (inserted by Commission Directive 93/21/EEC of 27 April, 1993) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex III" means Annex III (inserted by Commission Directive 93/21/EEC of 27 April, 1993) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex IV" means Annex IV (inserted by Commission | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(1) O.J. L154, 5.6.92 p.1 and O.J. L154A, 5.6.92 p.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) O.J. No. L383, 29.12.92 p.113 and O.J. L383A, 29.12.92 p.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) O.J. No. L110, 4.5.93 p.20 and O.J. L110A, 4.5.93 p.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) O.J. No. L227 8.9.93 p.9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) O.J. No. L258 16.10.93 p.29 and O.J. L258A, 16.10.93 p.1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) O.J. No. L277, 10.11.93 p33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) O.J. No. L13, 15.1.94 p.1 and O.J. L13A, 15.1.94 p.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) O.J. No. L294, 30.11.93 p21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(9) O.J. No. L314, 16.12.93 p38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(10) O.J. No. 196, 16.8.67 p.1-67 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex IV" means Annex IV (inserted by Commission Directive 93/21/EEC of 27 April, 1993) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex V" means Annex V (inserted by Commission Directives 88/302/EEC of 18 November, 1987(11), 92/69/EEC of 31 July, 1992, and 93/21/EEC of 27 April, 1993) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex VI" means Annex VI (inserted by Commission Directive 93/21/EEC of 27 April, 1993) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annexes VII.A, VII.B, VII.C and VII.D" means Annexes VII.A, VII.B, VII.C and VII.D (inserted by Council Directive 92/32/EEC of 30 April, 1992 and Commission Directive 93/105/EC of 25 November, 1993) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex VIII" means Annex VIII (inserted by Council Directive 92/32/EEC of 30 April, 1992) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Annex IX" means Annex IX (inserted by Commission Directive 91/410/EEC of 22 July, 1991(12)) to Council Directive 67/548/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"child-resistant fastening" means the cap, lid, fastening or other means of fastening a package, which complies with the provisions of Part A of Annex IX; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"the competent authority" has the meaning assigned to it by Regulation 5 of these Regulations; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"dossier" means a "notification dossier"; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"EINECS" means the European Inventory of Existing Commercial Substances(13) containing the definitive list of all substances deemed to be on the market in the European Communities on 18 September, 1981; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Elincs" means the European List of Notified Chemical Substances(14), published from time to time containing the list of substances placed on the market in the European Communities after 18 September, 1981 and which have been the subject of a notification; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(11) O.J. No. L133, 30.5.88 p.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(12) O.J. No. L228, 17.8.91 p.67-68 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(13) O.J. No. C146A, 15.6.90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(14) O.J. No. C130, 10.5.93 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
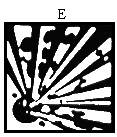
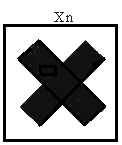
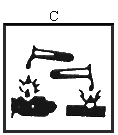
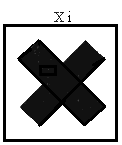
"indication of danger" means the indication of danger specified in Column 3 of Schedule 4 and required to be contained on the label or marked on the package of a dangerous substance in accordance with Regulation 19; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"inspector" means a person appointed by the Minister to be an inspector for the purpose of these Regulations; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"label" means the label required by Regulations 19 and 20; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"Minister" means the Minister for Enterprise and Employment; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"monomer unit" means the reacted form of a monomer in a polymer; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"notification" means the documents, with the requisite information, presented to the competent authority of a Member State; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"notifier" means the person submitting a notification; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"package" means the packaging, receptacle or container containing a substance, and "packaging" shall be construed accordingly; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"person responsible for placing on the market a substance to which these Regulations apply" includes a manufacturer, importer, supplier, distributor, wholesaler or retailer established in the State, who places on the market a substance to which these Regulations apply; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"placing on the market" means the making available to third parties, and importation into the European Communities customs territory shall be deemed to be placing on the market for the purposes of these Regulations; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"polymer" means a substance consisting of molecules characterized by the sequence of one or more types of monomer units and comprising a simple weight majority of molecules containing at least three monomer units which are covalently bound to at least one other monomer unit or other reactant, and consisting of less than a simple weight majority of molecules of the same molecular weight, such molecules being distributed over a range of molecular weights wherein differences in the molecular weight are primarily attributable to differences in the number of monomer units; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"preparations" means mixtures or solutions composed of two or more substances; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"process-oriented research and development" means the further development of a substance in the course of which pilot plant or production trials are used to tests the fields of application of the substance; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"risk phrase" means a phrase, listed in Annex III (which is set out in Schedule 2 hereto); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"safety phrase" means a phrase, listed in Annex IV (which is set out in Schedule 3 hereto); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"scientific research and development" means scientific experimentation, analysis or chemical research carried out under controlled conditions and includes the determination of intrinsic properties, performance and efficacy as well as scientific investigation related to product development; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"sole representative" means the person established in the European Communities who is so designated by the manufacturer of a substance manufactured outside the European Communities for the purposes of submitting a notification for that substance placed on the market, either on its own or in a preparation; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"substance" means a chemical element and its compounds in the natural state or obtained by any production process, including any additive necessary to preserve the stability of the product and any impurity deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"symbol" means any symbol specified in Annex II (which is set out in column 2 of Schedule 4); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
"tactile warning of danger" means a method of warning a person who has poor sight or no sight of the dangerous contents of a package referred to in Regulations 18, and which complies with the provisions of Part B of Annex IX. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) For the purposes of these Regulations the following are dangerous— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) explosive substances and preparations, namely, solid, liquid, pasty or gelatinous substances and preparations which may react exothermically without atmospheric oxygen thereby quickly evolving gases, and which, under defined test conditions, detonate, quickly deflagrate or upon heating explode when partially confined; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) oxidising substances and preparations, namely, substances and preparations which gave rise to a highly exothermic reaction in contact with other substances, particularly flammable substances; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) extremely flammable substances and preparations, namely, liquid substances and preparations having an extremely low flash-point and a low boiling point and gaseous substances and preparations which are flammable in contact with air at ambient temperature and pressure; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) highly flammable substances and preparations, namely: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) substances and preparations which may become hot and finally catch fire in contact with air at ambient temperature without any application of energy, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) solid substances and preparations which may readily catch fire after brief contact with a source of ignition and which continue to burn or to be consumed after removal of the source of ignition, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iii) liquid substances and preparations having a very low flash-point, or | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iv) substances and preparations which, in contact with water or damp air, evolve highly flammable gases in dangerous quantities; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( e ) flammable substances and preparations, namely, liquid substances and preparations having a low flash-point; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( f ) very toxic substances and preparations, namely, substances and preparations which in very low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( g ) toxic substances and preparations, namely, substances and preparations which in low quantities cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( h ) harmful substances and preparations, namely, substances and preparations which may cause death or acute or chronic damage to health when inhaled, swallowed or absorbed via the skin; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( i ) corrosive substances and preparations, namely, substances and preparations which may, on contact with living tissues, destroy them; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( j ) irritant substances and preparations, namely, non-corrosive substances and preparations which, through immediate, prolonged or repeated contact with the skin or mucous membrane, may cause inflammation; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( k ) sensitising substances and preparations, namely, substances and preparations which, if they are inhaled or if they penetrate the skin, are capable of eliciting a reaction of hypersensitisation such that on further exposure to the substance or preparation, characteristic adverse effects are produced; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( l ) carcinogenic substances and preparations, namely, substances or preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce cancer or increase its incidence; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( m ) mutagenic substances and preparations, namely, substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce heritable genetic defects or increase their incidence; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( n ) substances and preparations which are toxic for reproduction, namely, substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may produce, or increase the incidence of, non-heritable adverse effects in the progeny or an impairment of male or female reproductive functions or capacity; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
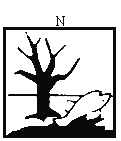
( o ) substances and preparations which are dangerous for the environment, namely, substances and preparations which, were they to enter the environment, would present or may present an immediate or delayed danger for one or more components of the environment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) ( a ) In these Regulations a reference to a Regulation or a Schedule is to a Regulation of, or to a Schedule to, these Regulations, unless it is indicated that reference to some other enactment is intended. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) In these Regulations a reference to a paragraph or subparagraph is to the paragraph or subparagraph of the provision in which the reference occurs, unless it is indicated that reference to some other provision is intended. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) Any requirement in these Regulations in relation to a substance shall apply to a substance to which these Regulations apply, unless otherwise indicated. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) A word or expression that is used in these Regulations and is also used in Council Directive 92/32/EEC has, unless the contrary intention appears, the same meaning in these Regulations as it has in that Directive. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
3 Construction and Application | 3. (1) The Safety, Health and Welfare at Work Act, 1989 , shall be construed and have effect as if these Regulations were existing enactments within the meaning of that Act and for the time being in force and specified in Part II to the Second Schedule of that Act. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) These Regulations apply to all substances, which are intended to be placed on the market either on their own or in a preparation, unless exempted under Regulation 4. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) The testing and notification requirements of these Regulations apply to substances not listed in the EINECS, which are intended to be placed on the market either on their own or in a preparation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) The classification requirements of these Regulations apply to all dangerous substances which are intended to be placed on the market. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) The packaging, labelling and safety data sheet requirements of these Regulations apply to substances, classified as dangerous under these Regulations, which are placed on the market. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
4 Exemptions. | 4. (1) These Regulations shall not apply to the following preparations in the finished state, intended for the final user— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) medicinal products for human or veterinary use, as defined in Directive 65/65/EEC(15), (as amended by Directive 87/21/EEC(16)); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) cosmetic products, as defined by Directive 76/768/EEC(17) (as amended by Directive 86/199/EEC(18)); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) mixtures of substances which, in the form of waste, are the subject of Directives 75/442/EEC(19) and 78/319/EEC(20) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) foodstuffs; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(e) animal feeding stuffs; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(15) O.J. No. 22, 9.2.65 p.369 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(16) O.J. No. L15, 17.1.87 p.36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(17) O.J. No. L262, 27.9.76 p.169 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(18) O.J. No. L149, 3.6.86 p.38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(19) O.J. No. L194, 15.7.75 p39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(20) O.J. No. L84, 31.3.78 p.43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(f) pesticides; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(g) radioactive substances, as defined by Directive 80/836/EEC(21); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(h) other substances or preparations for which European Communities notification or approval procedures exist and for which requirements relating to notification or approval are equivalent to those required by these Regulations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) These Regulations shall not apply to— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) the carriage of dangerous substances by rail, road, inland waterway, sea or air; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) substances in transit which are under customs supervision, provided they do not undergo any treatment or processing. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) The following substances shall be exempt from the notification requirements specified in these Regulations— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) substances which appear on the EINECS inventory; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) additives and substances for exclusive use in animal feeding stuffs and to which Directives 70/524/EEC(22) and 82/471/EEC(23) apply; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) substances used exclusively as additives in foodstuffs, to which Directive 89/107/EEC(24) applies, and substances used exclusively as flavourings in foodstuffs to which Directive 88/388/EEC(25) applies; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) active ingredients used exclusively in medicinal products for human or veterinary use, as defined in Directive 65/65/EEC, as amended by Directive 87/21/EEC; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(e) substances for exclusive use in other product sectors for which European Communities notification or approval procedures exist, as defined in Directive 93/90/EEC, and for which the requirements for data submission are equivalent to those specified in these Regulations; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(21) O.J. No. L246, 17.9.80 p1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(22) O.J. No. 1270. 14.12.70 p.1-15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(23) O.J. No. L213, 21,7.82 p8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(24) O.J. No. L40, 11.2.89 p27 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(25) O.J. No. L184, 15.7.88 p.61-66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(f) substances manufactured in the European Communities, which are not included in EINECS or Elincs but are intended solely for export outside the European Communities, provided the manufacturer notifies such substances to the competent authority, and in relation to which such notification includes the following information— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) the identity of the substance in accordance with point 1 of Annex VII. C, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) the quantity of the substance to be exported, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iii) the country of destination of the substance, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iv) information on the hazards of the substance, where available, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(v) the proposed labelling of the substance; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) The following substances shall be considered as having been notified within the meaning of these Regulations: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) polymers, with the exception of those which contain in combined from 2% or more of any substance which is not on EINECS; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) substances placed on the market in quantities of less than 10 kg per year per manufacturer; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) substances placed on the market in quantities not exceeding 100 kg per manufacturer per year, and intended solely for purposes of scientific research and development carried out under controlled conditions, provided the manufacturer or importer maintains written records, containing the identity of the substance labelling data, quantities and a list of customers involved. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) without prejudice to the provisions of paragraph (14) (a), substances placed on the market for the purposes of process-orientated research and development with a limited number of registered customers in quantities which are limited to the purpose of process-orientated research and development. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) (a) The packaging and labelling requirements of Regulations 18 to 20 shall not apply to munitions and explosives placed on the market with a view to producing a practical effect by explosion or a pyrotechnic effect. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) The packaging and labelling requirements of Regulations 18 to 20 shall not apply to butane, propane or liquified petroleum gas until April 30th, 1997. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) For the purpose of these Regulations, labelling requirements shall be deemed to be satisfied— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) in the case of an outer package containing one or more inner packages, if the outer package is labelled in accordance with international rules on the transport of dangerous substances and the inner package or packages are labelled in accordance with Regulations 19 and 20; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) in the case of a single package if such a package is labelled in accordance with international rules on the transport of dangerous substances and with Regulations 19 (1) (a), (b), (d), (e) and(f)and where appropriate, for particular types of packaging such as mobile gas cylinders, in accordance with the specific requirements referred to in Annex VI. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) The packaging of dangerous substances which are not explosive, very toxic or toxic may be unlabelled or may be labelled in such other way as may be approved by the competent authority if they contain such small quantities that there is no reason to fear any danger to persons handling such substances or to other persons. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) The packaging of dangerous substances which are explosive, very toxic or toxic may be labelled in such other way as may be approved by the competent authority if they are too small for labelling in accordance with Regulations 19 and 20 and there is no reason to fear any danger to persons handling such substances or to other persons. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(9) The labelling required on packages which are either too small or otherwise unsuitable for labelling in accordance with Regulations 19 and 20 may be applied on packages in such other appropriate manner as may be approved by the competent authority. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(10) A derogation under paragraphs (7), (8), or (9) shall not permit the use of symbols, indications of danger, risk phrases or safety phrases different from those required by these Regulations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(11) Where dangerous substances do not leave the State, labelling may be permitted by the competent authority which complies with national rules relating to the transport of dangerous substances. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(12) The competent authority may communicate the contents of notifications received under paragraph (3) (f) to the Commissioner and the competent authorities of other Member States. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(13) Records kept under paragraph (4) (c) shall be made available upon request to the competent authority where the manufacture, importation or scientific research and development takes place in the State. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(14) (a) Substances referred to in paragraph (4) (d) shall qualify for an exemption for a period of one year provided that the manufacturer or importer communicates their identity, labelling data, quantity, the justificiation for the quantity and a list of customers and the research and development programme to the competent authority and complies with any conditions imposed by the competent authority, and the condition imposed by the competent authority may include information not exceeding that provided for in Annex VII. C for quantities less than 500 kg, Annex VII. B for quantities between 500 kg and less and 1 tonne, Annex VII. A for other quantities. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) The manufacturer or importer shall also give an assurance to the competent authority that the substance or the preparation in which it is incorporated will be handled only by customers' staff in controlled conditions and will not be made available to the general public at any time either on its own or in a preparation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) Where the competent authority considers that there may exist an unacceptable risk for man and the environment, it may restrict any product, containing a new substance, which was produced during the process-orientated research and development. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) The one-year exemption period referred to in subparagraph (a) may be extended for a further year where the notifier can demonstrate, to the satisfaction of the competent authority that such an extension is justified. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(15) (a) A substance referred to in paragraph (4) shall, where the manufacturer may reasonably be expected to be aware of its dangerous properties, be packaged and provisionally labelled by the manufacturer or his representative in accordance with Regulations 18 to 20. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) Where it is not possible to label the substances completely and in accordance with Regulation 19, because the results of all tests provided for in Annex VII. A are not available, the label shall bear, in addition to the label deriving from the tests already carried out, the warning "Caution — substance not yet fully tested". | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) Where a substance referred to in paragraph (4) is labelled as very toxic, toxic, carcinogenic, toxic for reproduction, or mutagenic in accordance with Regulation 19, the manufacturer or importer of such a substance shall submit to the competent authority any appropriate information specified in Annex VII.A, Sections 2.3, 2.4 and 2.5, including, where available, acute toxicity data. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(16) Where the information specified in Annexes VII.A, VII.B, VII.C or VII.D is required in accordance with these Regulations a notifier need only supply items 1 and 2 of the relevant Annex in the case of a substance for which the information was originally submitted at least 10 years previously. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
5 Competent Authority | 5. The competent authority shall be the National Authority for Occupational Safety and Health established by Part III of the Safety, Health and Welfare at Work Act, 1989 . | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6 Placing on the Market | 6. (1) A person shall not place on the market a substance to which these Regulations apply either on its own or in a preparation unless it has been notified, packaged and labelled, and safety data sheets provided in accordance with these Regulations. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) The notification referred to in paragraph (1) shall, for substances manufactured in the State, be submitted to the competent authority by the manufacturer concerned. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) The notification referred to in paragraph (1) shall, for substances manufactured outside the European Communities, be submitted to the competent authority, by any person established in the State who is responsible for placing the substance either on its own or in a preparation on the market, or by the person established within the State who is, for the purpose of submitting a notification for a given substance placed on the market, either on its own or in a preparation, designated by the manufacturer as his sole representative. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) Paragraph (1) shall not apply to a substance placed on the market in quantities of less than one tonne per annum for any manufacturer which was notified under the European Communities (Dangerous Substances) (Classification, Packaging, Labelling and Notification) Regulations, 1982 ( S.I. No. 258 of 1982 ); provided that— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) the substance has been notified in the State prior to the commencement of these Regulations, and | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) the substance concerned has been manufactured by the same manufacturer of the substance to which the notification referred to in subparagraph (a) relates. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
7 Testing and Assessment | 7. (1) For the purpose of these Regulations— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) tests on substances shall as a general principle be conducted according to the methods laid down in Annex V; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) the physico-chemical properties of substances shall be determined according to the methods specified in Annex V (A); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) the toxicity of substances shall be determined according to the methods specified in Annex V (B); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) the ecotoxicity of substances shall be determined according to the methods specified in Annex V (C); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(e) laboratory tests on substances shall be carried out in compliance with the principles of good laboratory practice provided for in Directive 87/18/EEC(26) and in Directive 86/609/EEC(27); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(f) for substances on the EINECS the adequacy of the data for the purposes of classification and labelling and the need to conduct new tests shall be decided on a case-by-case basis taking into account the need to minimise testing on vertebrate animals. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) (a) Where more than one notification exists for a substance manufactured by the same manufacturer outside the European Communities, the obligation to carry out supplementary testing required under these Regulations will fall collectively on all notifiers placing that substance on the market; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) For substances referred to in subparagraph (a), if the quantities detailed in Regulation 10 (3) are attained, the competent authority shall contact each notifier and inform him of the identity of the other notifiers and shall draw his attention to the provisions of subparagraph (a). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Where it is necessary in the opinion of the competent authority for the purposes of carrying out the evaluation of the risks which may be caused by a substance in accordance with Directive 93/67/EEC, the competent authority may ask for further information, verification or confirmatory tests concerning the substances or their transformation products of which they have been notified or have received information under these Regulations and may also request information referred to in Annex VIII earlier than provided for in Regulation 10 (3). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(26) O.J. No. L15, 17.1.87 p.29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(27) O.J. No. L398, 18.12.86 p.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
8 Classification | 8. (1) For the purposes of these Regulations a dangerous substance shall be classified as one or more of the following— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) explosive; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) oxidising; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) extremely flammable; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) highly flammable; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(e) flammable; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(f) very toxic; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(g) toxic; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(h) harmful; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) corrosive; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(j) irritant; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(k) sensitising; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(l) carcinogenic; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(m) mutagenic; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(n) toxic for reproduction; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(o) dangerous for the environment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) In classifying a substance account shall be taken of the concentration of any impurity in as far as the latter exceeds the concentration limits specified in Annex I and in Article 3 of Directive 88/379/EEC(28). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(28) O.J. No. L187, 16.7.88 p.14-30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Dangerous substances shall be classified and labelled in accordance with the criteria in Annex VI and the results of tests provided for in Annexes VII.A, VII.B, VII.C, VII.D and VIII, except where contrary requirements for dangerous preparations are specified in the European Communities (Classification, Packaging and Labelling of Dangerous Preparations) Regulations, 1992 ( S.I. No. 393 of 1992 ). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
9 Obligation to carry out investigation | 9. Manufacturers, distributors and importers of dangerous substances, which appear in the EINECS but which have not yet been introduced into Annex I, shall carry out an investigation to make themselves aware of the relevant and accessible data existing concerning the properties of such substances, and on the basis of this information shall package and provisionally label these substances in accordance with Regulations 18 to 20. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
10 Full Notification | 10. (1) Subject to Regulation 4, a notifier intending to place on the European Communities market a substance in quantities of greater than or equal to one tonne per annum per manufacturer shall submit to the competent authority, a notification including— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) a technical dossier supplying the information necessary for evaluating the foreseeable risks, whether immediate or delayed, which the substance may entail for man and the environment, and containing all available relevant data for this purpose; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) a declaration concerning the unfavourable effects of the substance in relation to the various foreseeable uses; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) the proposed classification and labelling of the substance in accordance with these Regulations; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) in the case of dangerous substances, a proposal for a safety data sheet in accordance with Regulations 22; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(e) in the case of a manufacturer located outside the European Communities, a statement if appropriate from the manufacturer to the effect that, for the purpose of submitting a notification for the substance in question, he is designated as the manufacturer's sole representative; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( f ) if so desired by the notifier, a statement requesting on reasoned grounds that as a first notifier of a substance the notification be exempted from the provisions of Regulation 16 (2) for a maximum period which shall not in any case exceed one year following the date of notification; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( g ) if so desired by the notifier, a preliminary assessment of the real or potential risk to man and the environment on the basis of the principles adopted in Directive 93/67/EEC. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) A dossier required by paragraph (1) (a) shall contain the information and results of the studies referred to in Annex VII. A, together with a detailed and full description of the studies conducted and of the methods used or a bibliographical reference to them. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Notwithstanding anything in Regulation 15, a notifier of a substance already notified shall inform the competent authority— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) when the quantity of the substance placed on the market reaches 10 tonnes per year per manufacturer or when the total quantity placed on the market reaches 50 tonnes per manufacturer, and in such case the competent authority may require some or all of the additional tests and studies laid down in Annex VIII, level 1, to be carried out within such period as it determines; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) when the quantity of the substance placed on the market reaches 100 tonnes per year per manufacturer or when the total quantity placed on the market reaches 500 tonnes per manufacturer, and in such case the competent authority shall require the additional tests and studies laid down in Annex VIII, level 1, to be carried out within such period as it determines, unless the notifier can give good reason why a particular test or study is not appropriate or an alternative scientific test or study would be preferable; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) when the quantity of a substance placed on the market reaches 1,000 tonnes per year per manufacturer or when the total quantity placed on the market reaches 5,000 tonnes per manufacturer, and in such case the competent authority shall draw up a programme of tests and studies according to Annex VIII, level 2, to be carried out by the notifier within such period as the competent authority determines. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) When additional tests or studies are carried out in accordance with the requirements of paragraph (3) or voluntarily, the notifier shall provide the competent authority with the result of such tests or studies. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
11 Reduced Notification | 11. (1) Subject to Regulation 4, a notifier intending to place on the European Communities market a substance in quantities of less than one tonne per annum per manufacturer shall submit to the competent authority a notification including the information referred to in Regulation 10 (1). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) A dossier required by paragraph (1) shall contain the information and results of the studies referred to in Annex VII. B, together with a full and detailed description of the studies conducted and of the methods used or a bibliographical reference to them if the competent authority so requires. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) When the quantities to be placed on the market are below 100 kg per year per manufacturer the notifier may, without prejudice to Regulation 7 (3), restrict the information in the technical dossier referred to in paragraph (2) to that provided for in Annex VII.C. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) In the case of a notifier who has submitted a reduced notification dossier under paragraph (3), he shall, before the quantity of the substance placed on the market reaches 100 kg per year per manufacturer or before the total quantity placed on the market reaches 500 kg per manufacturer, provide the competent authority with the additional information necessary to complete the technical dossier to the level referred to in paragraph (2). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) In the case of a notifier who has submitted a reduced notification dossier under paragraph (1) he shall, before the quantity of the substance placed on the market reaches 1 tonne per year per manufacturer, or before the total quantity placed on the market reaches 5 tonnes per manufacturer, submit a full notification in accordance with Regulation 10. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) The substances notified under paragraphs (1) and (3) shall, in so far as the notifier may reasonably be expected to be aware of their dangerous properties, be packaged and provisionally labelled in accordance with Regulations 18 to 20. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) Where it is not possible to label substances in accordance with Regulation 19, because all the results of tests provided for in Annex VII.A are not available, the label shall bear, in addition to the label deriving from the tests already carried out, the warning "Caution — substance not yet fully tested". | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
12 Substances notified at least 10 years previously | 12. Where the information specified in Annexes VII.A, VII.B, VII.C or VII.D is required in accordance with these Regulations, a notifier need only supply items 1 and 2 of the relevant Annex in the case of a substance for which the information was originally submitted at least 10 years previously. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
13 Notification of Polymers | 13. In the case of polymers, the provisions concerning the technical dossiers contained in the notifications referred to in Regulations 10 (2) and 11 (2) shall be construed as if the reference to Annex VII. A and Annex VII.B respectively were a reference to Annex VII. D. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
14 Pre-marketing Notification Period | 14. (1) ( a ) Substances notified under Regulation 10 may, in the absence of any indication to the contrary from the competent authority, be placed on the market no sooner than 60 days after receipt by the competent authority of a dossier in conformity with the requirements of these Regulations. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) Where the competent authority considers that the dossier is not in conformity with these Regulations and advises the notifier accordingly, the substance may be placed on the market no sooner than 60 days after receipt by the competent authority of the information necessary to bring the notification into conformity with these Regulations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) ( a ) Substances notified under Regulation 11 (1) or Regulation 11 (3) may, in the absence of any indication to the contrary from the competent authority, be placed on the market no sooner than 30 days after receipt by the competent authority of a dossier in conformity with the requirements of these Regulations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) Where the competent authority considers that the dossier is not in conformity with these Regulations and advises the notifier accordingly, the substance may be placed on the market no sooner than 30 days after receipt by the competent authority of the information necessary to bring the notification into conformity with these Regulations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) Notwithstanding subparagraph (a) where the notifier has received notice from the competent authority of the official number which has been allocated to his notification indicating acceptance of the dossier, the substance may be placed on the market no sooner than 15 days after receipt of the dossier by the competent authority. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
15 Follow-up Information | 15. (1) Any notifier of a substance already notified in conformity with Regulation 10 (1) or Regulation 11 (1) shall inform the competent authority in writing of any— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) change in the annual or total quantities placed on the market by him or, by him or others as the case may be where he is the sole representative for a substance manufactured outside the European Communities; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) new knowledge of the effects of the substance on man of which he may reasonably be expected to have become aware; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) new knowledge of the effects of the substance on the environment of which he may reasonably be expected to have become aware; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) new uses for which the substance is placed on the market of which he may reasonably be expected to have become aware; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( e ) change in the composition of the substance as given in Annex VII.A, VII.B, VII.C or VII.D, section 1.3; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( f ) change in his status as manufacturer or importer. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) An importer of a substance produced by a manufacturer established outside the European Communities who imports the substance under a notification previously submitted by a sole representative shall ensure that the sole representative is provided with up-to-date information concerning the quantities of the substance placed on the market by him. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Where the sole representative ceases to act in that capacity the importer shall not submit the information required under Regulation 15 (2) to such other sole representative as may exist or, in his absence, to the competent authority. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
16 Re-notification | 16. (1) In the case of substance which has already been notified in accordance with Regulation 10 (1) or Regulation 11(1), the competent authority may agree that the subsequent notifier of that substance may, for the purposes of sections 3, 4 and 5 of Annexes VII.A, VII.B and VII.D and sections 3 and 4 of Annex VII. C, refer to the results of the tests and studies forwarded by the first notifier, in so far as the subsequent notifier can provide evidence that the substance renotified is the same as the one previously notified, including the degree of purity and the nature of impurities, but the first notifier shall give his agreement in writing to the references to the results of the tests and studies he has forwarded before such reference can be made. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) Before carrying out testing on vertebrate animals for the purpose of submitting a notification under Regulation 10 (1) or Regulation 11 (1) and notwithstanding paragraph (1), prospective notifiers shall enquire of the competent authority as to— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) whether or not the substance they intend to notify has already been notified; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) the name and address of the first notifier, and | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
this enquiry shall be supported by evidence that the prospective notifier intends to place the substance on the market and, specify the quantities involved. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Where a substance has been previously notified and the competent authority is satisfied with the evidence provided under paragraph (2), it shall supply the prospective notifier with the name and address of the first notifier and shall inform the first notifier of the name and address of the prospective notifier except where the first notifier has requested and been granted a temporary exemption from the provisions of this paragraph. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) The first notifier and the prospective notifier shall take all reasonable steps to reach an agreement on the sharing of information so as to avoid the duplication of testing on vertebrate animals. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) Notifiers of the same substance who have agreed to share information relating to Annexes VII.A, VII.B, VII.C or VII.D in accordance with paragraphs (1) and (4) shall take all necessary steps to reach an agreement on the sharing of information derived from testing on vertebrate animals submitted in conformity with Regulation 10 (3). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) Where notifiers and prospective notifiers of the same substance cannot reach an agreement on the sharing of data, the competent authority may require notifiers and prospective notifiers to share the data with a view to avoiding duplicative testing on vertebrate animals and may determine the procedure for utilising information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
17 Confidentiality of data | 17. (1) In the case of submissions made under Regulations 10, 11 or 15 the notifier may indicate the information required by these Regulations which he considers to be commercially sensitive and disclosure of which might harm him industrially or commercially, and which he wishes to be kept secret from all persons other than the competent authority and the Commission, and shall give full justification in such cases. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) Industrial and commercial secrecy shall not apply to— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) the trade name of the substance; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) the name of the manufacturer and the notifier; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) physico-chemical data concerning the substance in connection with section 3 of Annexes VII. A, VII.B, VII.C and VII.D; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) the possible ways of rendering the substance harmless; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( e ) the summary results of the toxicological and eco-toxicological tests; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( f ) the degree of purity of the substance or the identity of impurities or additives which are known to be dangerous within the meaning of these Regulations, if essential to classification and labelling for the purpose of introducing the substance into Annex I; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( g ) the recommended methods and precautions referred to in Annexes VII.A, VII.B, VII.C, and VII.D, section 2.3, and the emergency measures referred to in Annex VII.A, VII.B, VII.C and VII.D, sections 2.4 and 2.5; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( h ) the information contained in the safety data sheet; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( i ) in the case of substances in Annex I, analytical methods that make it possible to detect a dangerous substance when discharged into the environment as well as to determine the direct exposure of humans. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Where the notifier, manufacturer or importer himself subsequently discloses previously confidential information, he shall inform the competent authority accordingly. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) The competent authority, on receipt of information under Regulation 10, 11 or 15 shall decide at its discretion which information is covered by industrial and commercial secrecy in accordance with paragraph (1). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) Confidential information brought to the attention of the competent authority shall be kept secret by it. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) In all cases such confidential information— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) may be brought to the attention of the Commission and the competent authority of another Member State; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) may, when administrative or legal proceedings involving sanctions are undertaken for the purpose of controlling substances placed on the market, be divulged to persons directly involved in such proceedings. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) may be divulged to persons directly involved in providing medical information in the case of exposure or likely exposure of persons to the substance, especially in emergencies, and such information may only be used to formulate preventative and curative measures in relation to exposure of persons to the substance. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) For a substance appearing in Elincs which is not classified as dangerous for the purpose of these Regulations, its name may be included in the form of its trade name when requested by the competent authority. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) Substances referred to in paragraph (7) may be included in Elincs in the form of their trade name for a maximum of three years unless the competent authority considers that the publication of the chemical name in the International Union of Pure and Applied Chemistry (IUPAC) nomenclature itself could reveal information concerning commercial exploitation or manufacture, in which case the name of the substance may be recorded under its trade name alone for as long as the competent authority sees fit. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(9) Dangerous substances to which these Regulations apply may, at the request of the competent authority, be entered on Elincs in the form of their trade names alone until such time as they are introduced into Annex I. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
18 Packaging | 18. A dangerous substance to which these Regulations apply shall not be placed on the market unless its packaging satisfies the following requirements— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) it is so designed and constructed that its contents cannot escape, except in a case where special safety devices are prescribed by Regulations made by the Minister; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) the materials constituting the packaging and fastening are not susceptible to adverse attack by the contents, or liable to form dangerous compounds with the contents; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) the packaging and fastenings are sufficiently strong and solid throughout to ensure that they will not loosen and will safely meet the normal stresses and strains of handling; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) containers fitted with replaceable fastening devices can be repeatedly refastened without the contents escaping; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( e ) containers, containing dangerous substances which are offered or sold to the general public, and which are labelled "very toxic", "toxic" or "corrosive" as defined in these Regulations must have a child resistant fastening and bear a tactile warning of danger; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( f ) containers, containing dangerous substances which are offered or sold to the general public, and which are labelled "harmful", "extremely flammable" or "highly flammable" as defined in these Regulations must bear a tactile warning of danger. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
19 Labelling | 19. (1) A dangerous substance to which these Regulations apply shall not be placed on the market unless the labelling on its packaging shows clearly and indelibly the following— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) the name of the substance under one of the designations given in Annex I, but where the substance is not yet listed in Annex I, a name using an internationally recognised designation; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) the name and full address, including the telephone number of the person established in the European Communities who is responsible for placing the substance on the market; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) danger symbols, when laid down, and indication of the danger involved in the use of the substance; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) standard phrases (R-phrases) indicating the special risks arising from the dangers involved in using the substance; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( e ) standard phrases (S-phrases) relating to the safe use of the substance; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( f ) the EEC number, when allocated; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( g ) for substances appearing in Annex I, the label shall also include the words "EEC label". | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) ( a ) The design of a danger symbol and the wording of the indications of danger shall comply with those laid down in Annex II. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) The symbol shall be printed in black on an orange-yellow background. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) The symbols and indications of danger to be used for each substance shall be those indicated in Annex I. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) For dangerous substances not yet appearing in Annex I the danger symbols and indications of danger shall be assigned according to the rules laid down in Annex VI. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( e ) When more than one danger symbol is assigned to a substance the obligation to indicate: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) the symbol T makes the symbols X and C optional, unless Annex I provides otherwise, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) the symbol C makes the symbol X optional, and | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iii) the symbol E makes the symbol F and O optional. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) ( a ) The wording of R-phrases shall comply with that laid down in Annex III. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) The R-phrases to be used for each substance shall be as indicated in Annex I. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) For dangerous substances not yet appearing in Annex I the R-phrases to be used shall be assigned according to the rules laid down in Annex VI. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) ( a ) The wording of S-phrases shall comply with that laid down in Annex IV. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) The S-phrases to be used for each substance shall be as indicated in Annex I. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) For dangerous substances not yet appearing in Annex I, the S-phrases to be used shall be assigned according to the rules laid down in Annex VI. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) The EEC number shall be obtained from the EINECS or from the Elincs. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) ( a ) In the case of irritant, highly flammable, flammable and oxidizing substances, an indication of R-phrases and S-phrases need not be given where the package does not contain more than 125 millilitres of the substance. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) This paragraph shall also apply in the case of the same volume of harmful substances not retailed to the general public. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) Indications such as "non-toxic", "non-harmful" or any other similar indications shall not appear on the label or packaging of substances to which these Regulations apply. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) The information referred to in paragraph (1) shall be shown on the packaging in the English language or in both the English and Irish languages. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
20 Implementation of labelling | 20. (1) Where the particulars required by Regulation 19 appear on a label, that label shall be firmly affixed to one or more surfaces of the packaging so that these particulars can be read horizontally when the package is set down normally. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) The dimensions of such a label shall be as follows— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Each symbol required by Regulation 19 shall cover at least one-tenth of the surface area of the label but not be less than one square centimetre (1 cm2), and the entire surface of the label shall adhere to the package immediately containing the substance; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) A label shall not be required where the particulars are clearly shown on the package itself in accordance with this Regulation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) The colour and presentation of the label (or, in the case of paragraph (3), of the package) shall be such that the danger symbol and its background stand out clearly from the label or package. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) The information required on a label by Regulation 19 shall stand out clearly from its background and shall be of such size and spacing as to be easily read and shall be in accordance with the provisions of Annex VI. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
21 Advertising | 21. A person shall not publish any advertisement for a substance which belongs to one or more of the categories referred to in Regulation 8 (1) ( a )-( o ) unless mention is made in the advertisement of the category or categories concerned. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
22 Safety Data Sheet | 22. (1) A person placing a dangerous substance to which these Regulations apply on the market shall prepare a safety data sheet giving information on that substance. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) A safety data sheet shall be amended by the person who provides it when any new information of a significant nature so requires. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) At, or before the first delivery, following the commencement of these Regulations, the manufacturer, importer or distributor of a dangerous substance to which these Regulations apply shall communicate the safety data sheet to any recipient who is a professional or industrial user, distributor, wholesaler or retailer of the substance. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) The safety data sheet referred to in paragraph (3) shall be provided free of charge to the recipient and may be communicated on paper or electronically. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) An amended safety data sheet referred to in paragraph (2) shall be provided forthwith free of charge to all industrial or professional users, distributors, wholesalers and retailers who were supplied with the particular dangerous substance within the 12 months preceding the publication date of the amended safety data sheet. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) Without prejudice to paragraphs (3) and (4), recipients or users of a dangerous substance shall on request be provided with the safety data sheet by the supplier of the substance to them. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) The safety data sheet referred to in paragraph (1) shall contain such information necessary for the protection of man and the environment as the manufacturer, importer or distributor may reasonably be expected to be aware of. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) A safety data sheet referred to in paragraph (1) shall be clearly written in the English language or in both the English and Irish languages. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(9) ( a ) A safety data sheet required to be provided in accordance with this Regulation shall contain information on the dangerous substance under the headings set out in Schedule I and shall contain those headings. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) The information required to be contained in the safety data sheet in accordance with subparagraph ( a ) shall be compiled in accordance with the guidelines laid down in the Annex to Directive 93/112/EEC(29). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) The information required under subparagraph ( a ) shall include— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) the name of the person responsible for providing the safety data sheet, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) the date of publication/preparation of the safety data sheet, and | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iii) for an amended safety data sheet, a notice of revision together with the revision date. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
23 Supply of Substances | 23. A notifier shall supply to the competent authority on request such quantities of a notified substance as the competent authority deems necessary for the carrying out of verification tests. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
24 Restriction on Sale | 24. Where the competent authority is of opinion that a substance, although satisfying the requirements of these Regulations, constitutes a hazard for man or the environment by reason of its classification, packaging or labelling, the competent authority may, by notice in writing to the person who placed the substance on the market, prohibit the sale of that substance or subject its placing on the market to special conditions. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(29)O.J. No. L314, 16.12.93 p.38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
25 Fees Payable by Notifier | 25. The fee fixed by column 2 of Schedule 5 shall be payable in advance by a notifier to the competent authority in relation to any matter referred to in the corresponding entry in column 1 of that Schedule. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
26 Taking and Detention of Substances | 26. (1) An inspector may, seize and retain, or seize, remove and retain any substance which he believes is a substance to which these Regulations apply and in relation to which he has reasonable grounds for suspecting that there is or has been a failure to comply with any provision of these Regulations. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) An inspector may, by a notice in writing given to the owner or to the person in apparent charge or control of a substance which has been seized under this Regulation— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) require things specified in the notice to be done in relation to the substance before it is released by an inspector; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) either— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) require the disposal of the substance by the person to whom the notice is given, in a manner specified in the notice and at the expense of the owner, or | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) indicate the inspector's intention of disposing of the substance at the expense of the owner, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
such disposal to be, in either case, such as will prevent the substance from being again placed on the market, and, where a notice given under this paragraph requires specified things to be done in relation to a substance, the inspector shall retain control of the substance to which the notice relates until the requirements of the notice have been complied with. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) Where a notice is given under this Regulation, a person shall not, without the consent of an inspector sell, move, dispose of or otherwise interfere with the substance in any way pending compliance with the requirements of the notice. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) Any person who is aggrieved by a notice given under paragraph (2) of this Regulation which either requires the substance to which it relates to be disposed of or indicates an intention to dispose of such substances may, not later than the expiration of the period of seven days beginning on the date of the notice, appeal to the appropriate court against the notice. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) ( a ) Where an appeal is made to the appropriate court under paragraph (4) the court, if it is satisfied that— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) the substance to which the relevant notice under this Regulation relates is one to which these Regulations apply, and | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) if such substance were released, it might be placed on the market, and | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iii) there has been a failure to comply with the provisions of these Regulations— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
may order that the substance be disposed of in the manner specified in the notice, or in such other manner as may be specified by the court which, in the opinion of the court, will prevent the substance from being placed on the market. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) Where an order made by a court under this paragraph requires the substance to which it relates to be disposed of by an inspector, the cost of such disposal shall be recoverable by the competent authority as a simple contract debt in any court of competent jurisdiction from the person who was the owner of the product at the time of its seizure under this Regulation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) A notice under this Regulation shall not come into force unless— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) where an appeal is taken against the notice, the appeal is withdrawn; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) in any other case, the period within which such an appeal may be taken has expired. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) In this Regulation 'appropriate court' means in relation to an appeal made under this Regulation against a notice given under paragraph (2)— | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) in case the estimated value of the substance and cost of complying with the order to which the appeal relates does not exceed £5,000, the District Court for the district in which the goods were seized; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) in case the estimated amounts aforesaid does not exceed £30,000, the Judge of the Circuit Court for the circuit in which the goods were seized; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) in any other case, the High Court. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) ( a ) If, in relation to an appeal under this Regulation to the District Court, that court becomes of the opinion during the hearing of the appeal that the estimates cost aforesaid will exceed £5,000, it may, if it so thinks fit, transfer the appeal to the Circuit Court or the High Court, whichever it considers appropriate having regard to the estimated cost aforesaid. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) If, in relation to an appeal under this regulation to the Circuit Court, that court becomes of opinion during the hearing of the appeal that the estimated amounts aforesaid will exceed £30,000, it may, if it so thinks fit, by order transfer the appeal to the High Court. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
27 Revocations | 27. The following are hereby revoked:— | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( a ) The European Communities (Dangerous Substances) (Classification, Packaging and Labelling) Regulations, 1979 ( S.I. No. 383 of 1979 ); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( b ) The European Communities (Dangerous Substances) (Classification, Packaging, Labelling and Notification) Regulations, 1982 ( S.I. No. 258 of 1982 ); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( c ) The European Communities (Dangerous Substances) (Classification, Packaging, Labelling and Notification) (Amendment) Regulations, 1985 ( S.I. No. 89 of 1985 ); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
( d ) The European Communities (Dangerous Substances) (Classification, Packaging, Labelling and Notification) Regulations, 1992 ( S.I. No. 426 of 1992 ). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SCHEDULE 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Regulation 22 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Obligatory Headings for Safety Data Sheets | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. Identification of the substance/preparation and of the company/undertaking; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2. Composition/information on ingredients; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. Hazards identification; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4. First-aid measures; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. Fire-fighting measures; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
6. Accidental release measures; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
7. Handling and storage; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
8. Exposure controls/personal protection; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
9. Physical and chemical properties; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
10. Stability and reactivity; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
11. Toxicological information; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
12. Ecological information; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
13. Disposal considerations; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
14. Transport information; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
15. Regulatory information; | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
16. Other information. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SCHEDULE 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
RISK PHRASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Regulation 19 (1) ( d ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. The risk phrase or phrases to be marked on the package of a dangerous substance or contained in the label in accordance with the provisions of these Regulations shall be selected in accordance with the provisions of Annex VI to Directive 67/548/EEC. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2. The risk phrase or phrases required to be marked on the package of a dangerous substance or contained in the label in accordance with the provisions of paragraph 1 shall conform to those contained in Annex III to Directive 67/548/EEC, as set out in the Tables below. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. In the case of a dangerous substance assigned two or more single risk phrases contained in Table A below in accordance with the provisions of paragraph 1, and which can be combined in a combined risk phrase contained in Table B below, that combined risk phrase shall be assigned to the dangerous substance in place of the single phrases assigned. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SINGLE RISK PHRASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COMBINED RISK PHRASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SCHEDULE 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SAFETY PHRASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Regulation 19 (1) (e) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. The safety phrase or phrases to be marked on the package of a dangerous substance or contained in the label shall be selected in accordance with the provisions of Annex VI to Directive 67/548/EEC. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2. The safety phrase or phrases required to be marked on the package of a dangerous substance or contained in the label in accordance with the provisions of paragraph 1 shall conform to those contained in Annex IV to Directive 67/548/EEC, as set out in the Tables below. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. Where two or more single safety phrases contained in Table A below are selected, in accordance with the provisions of paragraph 1, and which can be combined in a combined safety phrase contained in Table B below, that combined safety phrase shall be assigned to the dangerous substance in lieu of the single ones selected. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SINGLE SAFETY PHRASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COMBINED SAFETY PHRASES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SCHEDULE 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DANGER SYMBOLS AND INDICATIONS OF DANGER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Regulation 19 (1) (c) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. Any danger symbol or symbols and indication or indications of danger to be marked on the package of a dangerous substance or contained in the label in accordance with the provisions of Regulations 19(1) (c) and 20 (2) shall, subject to paragraph 3, be those assigned to the dangerous substance in accordance with the provisions of paragraph 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2. (a) Any danger symbol or symbols and indication or indications of danger assigned to a dangerous substance shall be that or those specified in the Table below, according to the classification or classifications of the dangerous substance, determined in accordance with the provisions of Regulation 8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) Each such danger symbol and indication of danger shall be that specified in column 2 and 3 respectively of the Table below, for each classification in column 1 of the Table below, of the dangerous substance. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. For the purposes of Regulation 19 (1) c, the characters specified in column 4 are those respectively for the danger symbols in column 2 of the Table below. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SCHEDULE 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FEES PAYABLE BY NOTIFIER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Regulation 25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note 1. Additional charge where notification particulars are not provided on an approved diskette — £100. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note 2. Additional charge where an adequate risk assessment is not included — £2,000. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note 3. Additional charge where an adequate risk assessment is not included — £500. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GIVEN under my Official Seal, this 7th day of April, 1994. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
RUAIRI QUINN, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Minister for Enterprise and Employment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EXPLANATORY NOTE. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
These Regulations implement Council Directive 92/32/EEC of 30th April 1992, amending for the 7th time Directive 67/548/EEC on the classification, packaging and labelling of dangerous substances, together with eight other associated Commission Directives. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
They apply to all substances which are intended to be placed on the market either on their own or in a preparation with the exception of certain categories of substances (medicinal, cosmetic, pesticide, waste, etc) which are covered by other Directives. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The aim of the Regulations is to protect man and the environment from the harmful effects of both new and existing dangerous substances. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
These regulations require each manufacturer, importer or other person proposing to place any new chemical on the market for the first time to submit to the competent authority a notification containing details of tests to which the substance has been subjected and the proposed classification and labelling of the substance. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Regulations require suppliers to supply safety data sheets and also to put warning labels on containers for dangerous substances and ensure that the containers are properly designed, constructed and secured to prevent spillage or seepage during normal use. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
These Regulations also implement Commission Directives which adapt to technical progress the Annexes to, or which expand some of the provisions of, Council Directive 67/548/EEC. These Directives are: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. Commission Directive 92/69/EEC of 31 July, 1992 which adapts to technical progress Annex V, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2. Commission Directive 93/21/EEC of 27 April, 1993 which adapts to technical progress Annex II, III, IV & VI, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. Commission Directive 93/67/EEC of 20 July, 1993 which lays down the principles for the risk assessment of substances notified under these Regulations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4. Commission Directives 93/72/EEC of 1 September, 1993, and 93/101/EC of II November, 1993, which adapt to technical progress Annex I, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. Commission Directive 93/90/EEC of 29 October, 1993 which provides a list of substances for which Community notification or approval procedures exist and which are exempt from the notification requirements of Council Directive 67/548/EEC, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
6. Commission Directive 93/105/EC of 25 November, 1993 which adapts to technical progress Annex VII.2 D, and | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
7. Commission Directive 93/112/EC of 10 December, 1993 which amends the guide to the compilation of safety data sheets for substances and preparations. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Four sets of existing Regulations dealing with the classification, packaging, labelling and notification of dangerous substances are revoked by these Regulations, as follows: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) The European Communities (Dangerous Substances) (Classification, Packaging and Labelling) Regulations, 1979 ( S.I. No. 383 of 1979 ); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) The European Communities (Dangerous Substances) (Classification, Packaging, Labelling and Notification) Regulations, 1982 ( S.I. No. 258 of 1982 ); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) The European Communities (Dangerous Substances) (Classification, Packaging, Labelling and Notification) (Amendment) Regulations, 1985 ( S.I. No. 89 of 1985 ); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) The European Communities (Dangerous Substances) (Classification, Packaging, Labelling and Notification) Regulations, 1992 ( S.I. No. 426 of 1992 ). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Hazardous Substance Assessment Unit of the Health and Safety Authority, 10 Hogan Place, Dublin 2 is the competent authority to which notifications under these Regulations should be sent. |