| |
(SI No 143 of 2007)
|
| |
EUROPEAN COMMUNITIES (CONTROL OF ANIMAL REMEDIES AND THEIR RESIDUES) REGULATIONS 2007
|
| |
I, Mary Coughlan, Minister for Agriculture and Food, in exercise of the powers conferred on me by section 3 of the European Communities Act, 1972 (No. 27 of 1972), for the purpose of giving effect to Council Directive 96/22/EC1
of 29 April 1996 and Council Directive 96/23/EC2
of 29 April 1996, as amended, hereby make the following Regulations:
|
|
|
Citation and Commencement
|
1. (1) These Regulations may be cited as the European Communities (Control of Animal Remedies and their Residues) Regulations, 2007.
|
| |
(2) These Regulations shall come into operation on the 2nd day of April 2007.
|
|
|
Interpretation
|
2. (1) In these Regulations -
|
| |
“the Act” means the Animal Remedies Act, 1993 (No. 23 of 1993);
|
| |
“animal” has the same meaning as it has in Section 2 of the Act and in the Animal Remedies Act (Section 2) Order 2005 (SI No 733 of 2005);
|
| |
“animal remedies authorisation” has the same meaning as it has in the European Communities (Animal Remedies) Regulations 2007;
|
| |
“approved laboratory” means a laboratory designated as an approved laboratory in accordance with Regulation 14;
|
| |
“authorised animal remedy” has the same meaning as it has in the European Communities (Animal Remedies) Regulations 2007;
|
| |
“authorised officer” means an authorised officer within the meaning of the Act;
|
| |
“Council Directives” means Council Directive 96/22/EC of 29 April 1996 and Council Directive 96/23/EC of 29 April 1996, as amended;
|
| |
“eartag” means an approved eartag within the meaning of the Bovine Tuberculosis (Attestation of the State and General Provisions) Order, 1989 (S.I. No. 308 of 1989), or an eartag referred to in Regulation 4 of the Bovine Tuberculosis (Attestation of the State and General Provisions) Order, 1996 (S.I. No. 103 of 1996), or an eartag referred to in Article 4 of Council Regulation (EC) No 1760/20003
of 17 July 2000
|
| |
“establishment” means an establishment approved under the European Communities (Food and Feed Hygiene) Regulations 2005 (S.I. No. 910 of 2005) used for the slaughter of animals;
|
| |
“identity card” means-
|
| |
(a) an identity card within the meaning of the Bovine Tuberculosis (Attestation of the State and General Provisions) Order 1989 (S.I. No. 308of 1989), or the Brucellosis in Cattle (General Provisions) Order 1991 (S.I. 114 of 1991), or
|
| |
(b) an animal passport to which Council Regulation (EC) 1760/2000 of 17 July 2000 relates;
|
| |
“manufacturer's licence” has the same meaning as it has in the European Communities (Animal Remedies) Regulations of 2007;
|
| |
“maximum residue limit” has the same meaning as it has in Council Regulation (EEC) No. 2377/904
of 26 June 1990 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin;
|
| |
“member state” means a member state of the European Communities;
|
| |
“national reference laboratory” means a laboratory designated as a national reference laboratory in accordance with Regulation 14;
|
| |
“official mark” means a mark in the form set out in the Schedule;
|
| |
“prescription” has the same meaning as it has in the European Communities (Animal Remedies) Regulations of 2007, and cognate words shall be construed accordingly;
|
| |
“registered veterinary practitioner” means a person registered in the Register of Veterinary Practitioners for Ireland;
|
| |
“slaughter” in relation to an animal, means slaughter for the purposes of the production of meat or other food intended for human or animal consumption.
|
| |
(2) A word or expression that is used in these Regulations and that is also used in the Council Directives has, unless the contrary intention appears, the same meaning in these Regulations as it has in the Council Directives.
|
| |
(3) A word or expression that is used in these Regulations and is also used in the Act has, in these Regulations, unless the contrary intention appears, the same meaning as it has in the Act.
|
|
|
Restrictions on Manufacture etc. of Certain Animal Remedies
|
3. (1) A person shall not manufacture, import, possess, sell or supply an animal remedy consisting of or containing a stilbene, stilbene derivative, salt or ester of a stilbene or stilbene derivative, or a thyrostatic substance.
|
| |
(2) Subject to the provisions of this Regulation, a person shall not manufacture, import, possess, sell or supply an animal remedy consisting of or containing -
|
| |
(a) a beta-agonist, or
|
| |
(b) a substance having an oestrogenic, androgenic or gestagenic action.
|
| |
(3) A person may, in accordance with an animal remedies authorisation and a manufacturer's licence, manufacture, or import from a state other than a member state, an animal remedy consisting of or containing a substance to which paragraph (2) applies.
|
| |
(4) A person may, in accordance with an animal remedies authorisation, possess, sell or supply, or import from a member state, an authorised animal remedy consisting of or containing a substance to which paragraph (2) applies.
|
| |
(5) A person shall not manufacture, import, sell, hire or otherwise supply or use, or have in his or her possession or under his or her control any plant, machinery, instrument, cartridge, container, utensil, label, package, package insert or other thing, made or adapted for use in connection with -
|
| |
(a) the manufacture of a substance, to which paragraph (1) applies, or a prohibited animal remedy,
|
| |
(b) the administration of a prohibited animal remedy to a farm or aquaculture animal, or
|
| |
(c) the administration of a substance, to which paragraph (1) applies, to a farm or aquaculture animal.
|
| |
(6) A person may, pursuant to a licence under Regulation 17 of the European Communities (Animal Remedies) Regulations 2007, import into the State an animal remedy within the meaning of that Regulation consisting of or containing a substance to which paragraph (2) applies.
|
| |
(7) This Regulation does not apply to a substance -
|
| |
(a) that is intended to be used for and capable, of being used for, purposes other than agricultural or veterinary purposes.
|
| |
(b) that is labelled as being intended for purposes other than agricultural or veterinary purposes, and
|
| |
(c) in respect of which there is for the time being in force a product authorisation under the Medical Preparations (Licensing and Sale) Regulations, 1996 (S.I. No. 43 of 1996).
|
|
|
Prohibition on Administration of Stilbenes etc.
|
4. (1) A person shall not administer or cause to be administered to a farm or aquaculture animal an animal remedy consisting of or containing a substance to which paragraph (1) of Regulation 3 applies.
|
| |
(2) A person shall not-
|
| |
(a) import, export, sell, supply or slaughter an animal to which an animal remedy referred to in paragraph (1) has been administered,
|
| |
(b) import, export, sell or supply any meat of, or other food derived from, an animal referred to in subparagraph (a), or any meat product prepared from or with any such meat,
|
| |
(c) subject meat or other food referred to in subparagraph (b) to any manufacturing process, or
|
| |
(d) be in possession of or have under his or her control an animal to which an animal remedy has been administered in contravention of paragraph (1), or meat, meat products or other food derived from such an animal.
|
|
|
Restrictions on Administration of Beta-agonists etc.
|
5. (1) Subject to Regulations 8, 9 and 10 a person shall not administer or cause to be administered to a farm or aquaculture animal an animal remedy consisting of or containing a substance to which paragraph (2) of Regulation 3 applies.
|
| |
(2) a person shall not-
|
| |
(a) import, export, sell, supply or slaughter an animal to which an animal remedy referred to in paragraph (1) has been administered,
|
| |
(b) import, export, sell or supply any meat of, or other food derived from, an animal referred to in subparagraph (a), or any meat product prepared from or with any such meat,
|
| |
(c) subject meat or other food referred to in subparagraph (b) to any manufacturing process, or
|
| |
(d) be in possession of or have under his or her control an animal to which an animal remedy has been administered in contravention of paragraph (1), or meat, meat products or other food derived from such an animal.
|
|
|
Unlawful to Sell or Slaughter certain Animals etc.
|
6. (1) A person shall not sell or slaughter a farm or aquaculture animal to which a prohibited animal remedy has been administered.
|
| |
(2) A person shall not sell or slaughter a farm or aquaculture animal where there is present in such animal a quantity of an animal remedy in excess of the maximum residue limit.
|
| |
(3) A person shall not sell meat, a meat product or other food of animal origin that contains an animal remedy consisting of or containing a substance to which Regulation 3(1) applies, or a quantity of an authorised animal remedy in excess of the maximum residue limit.
|
| |
(4) A person shall not sell meat, a meat product or other food of animal origin that contains a prohibited animal remedy.
|
|
|
Detention of Certain Animals at Establishments
|
7. (1) If an authorised officer has reasonable grounds for believing that a prohibited animal remedy or an animal remedy consisting of or containing a substance to which Regulation 3(1) applies has been administered to an animal presented for slaughter at an establishment he or she shall -
|
| |
(a) direct that the animal be slaughtered separately from other animals at the establishment at such time as he may specify, and
|
| |
(b) (i) issue a direction under Regulation 15 in respect of the meat, offal and carcase of the animal concerned, or
|
| |
(ii) detain the meat, offal and carcase of the animal concerned.
|
| |
(2) If an authorised officer has reasonable grounds for believing that an authorised animal remedy has been administered to an animal presented for slaughter at an establishment and that the withdrawal period in respect thereof has not expired he or she shall give a direction under Regulation 15 to the owner or occupier of the establishment concerned -
|
| |
(a) prohibiting the movement of the animal from the establishment, and
|
| |
(b) prohibiting the sale or slaughter of the animal,
|
| |
until such time as the authorised officer is satisfied that the quantity of the authorised animal remedy concerned present in the animal no longer exceeds the maximum residue limit.
|
| |
(3) A prohibition in a direction given under paragraph (2) shall remain in force for a period of not less than 28 days.
|
| |
(4) If having regard to the provisions of Article 24(2) of Council Directive 96/23/EC of 29 April 1996 it would not be practicable for an authorised officer to give a direction in accordance with paragraph (2) he or she may direct that the animal concerned be slaughtered before the expiration of the period specified in paragraph (3) and give a direction under Regulation 15 to the owner or occupier of the establishment concerned prohibiting the movement or sale of all meat, meat products or other food derived from such animal until such time as he or she is satisfied that it does not contain a quantity of an authorised animal remedy in excess of the maximum residue limit.
|
| |
(5) An authorised officer shall declare to be unfit for human consumption all meat, meat products or other food derived from an animal in which, at the time of its slaughter, there is present a quantity of an authorised animal remedy in excess of the maximum residue limit.
|
| |
(6) An authorised officer shall declare to be unfit for human consumption all meat, meat products or other food of animal origin containing a quantity of an authorised animal remedy in excess of the maximum residue limit.
|
|
|
Administration of certain Animal Remedies for Therapeutic Purposes
|
8. (1) A registered veterinary practitioner may, for therapeutic purposes, administer to a farm animal an authorised animal remedy consisting of or containing testosterone, progesterone. or derivatives thereof that readily yield the parent compound on hydrolysis after absorption at the site of application:
|
| |
Provided that -
|
| |
(a) (i) if it is intended to administer the authorised animal remedy for the purpose of treating ovarian dysfunction in the farm animal concerned, it is administered by vaginal spiral, or
|
| |
(ii) in all other cases, it is administered by injection, and
|
| |
(b) the animal remedy concerned is administered in accordance with the animal remedies authorisation in respect thereof.
|
| |
(2) A registered veterinary practitioner or, pursuant to a prescription, a person other than a registered veterinary practitioner, may, for therapeutic purposes, administer to an equid or companion animal an authorised animal remedy consisting of or containing allyl trenbolone, provided that it is administered orally and in accordance with the animal remedies authorisation in respect thereof.
|
| |
(3) A farm animal (other than a bovine animal to whose ear an eartag has been affixed) to which an authorised animal remedy has been administered in accordance with this Regulation shall be marked by the owner or person in charge of the farm animal at the time the animal remedy is administered in such manner as will enable it to be readily identified.
|
| |
(4) In this Regulation, “prescription” means a prescription issued by a registered veterinary practitioner in accordance with and in the form prescribed by Regulation 45 of the European Communities (Animal Remedies) Regulations 2007.
|
|
|
Administration of Beta-agonists
|
9. (1) A registered veterinary practitioner may, for the purpose of inducing tocolysis, administer to a cow in calf an authorised animal remedy consisting of or containing a beta-agonist, provided that the animal remedy concerned is administered by injection and in accordance with the animal remedies authorisation in respect thereof.
|
| |
(2) A registered veterinary practitioner or, pursuant to a prescription (within the meaning of Regulation 8), a person other than a registered veterinary practitioner, may, for the purpose of tocolysis or to treat a respiratory problem, administer, to equidae raised for purposes other than meat production or a companion animal, an authorised animal remedy consisting of or containing a beta-agonist, provided that the animal remedy concerned is administered in accordance with the animal remedies authorisation in respect thereof.
|
| |
(3) The owner or person in charge of a farm animal (other than a bovine animal to whose ear an eartag has been affixed) to which an authorised animal remedy has been administered in accordance with this Regulation shall mark the farm animal at the time the animal remedy is administered in such manner as will enable it to be readily identified.
|
| |
(4) In this Regulation, “companion animal” has the same meaning as it has in the European Communities (Animal Remedies) Regulations 2007.
|
|
|
Administration of certain Animal Remedies for Zootechnical Purposes
|
10. (1) A registered veterinary practitioner may, for zootechnical purposes, administer to a farm animal an authorised animal remedy consisting of or containing a substance having an oestrogenic (other than oestradiol 17β and its ester-like derivatives), androgenic or gestagenic action:
|
| |
provided that-
|
| |
(a) the farm animal concerned is deemed, for the purposes of Regulation 44 of the European Communities (Animal Remedies) Regulations 2007, to be under the care of the registered veterinary practitioner who administers the authorised animal remedy,
|
| |
(b) the registered veterinary practitioner is deemed, for the purposes of the said Regulation 44 to have been consulted in a professional capacity regarding the care of the farm animal concerned, and
|
| |
(c) the animal remedy is administered in accordance with the animal remedies authorisation in respect thereof.
|
| |
(2) A registered veterinary practitioner or, pursuant to a prescription, a person other than a registered veterinary practitioner, may, in accordance with an animal remedies authorisation, administer an authorised animal remedy consisting of or containing a substance having an oestrogenic (other than oestradiol and its ester-like derivatives), androgenic or gestagenic action to a farm animal for the purposes of synchronising oestrus and preparing donors and recipients for the implantation of embryos.
|
| |
(3) A registered veterinary practitioner or, pursuant to a prescription, a person other than a registered veterinary practitioner, may, in accordance with an animal remedies authorisation, administer an authorised animal remedy consisting of or containing a substance having an androgenic action to an aquaculture animal of not more than 3 months in age for the purposes of sex inversion.
|
| |
(4) The owner or person in charge of a farm animal (other than a farm animal to whose ear an eartag has been affixed) to which an authorised animal remedy has been administered in accordance with this Regulation shall mark the animal at the time the animal remedy is administered in such manner as will enable the farm animal to be readily identified.
|
| |
(5) In this Regulation, “prescription” means a prescription issued by a registered veterinary practitioner in accordance with Regulation 45 of the European Communities (Animal Remedies) Regulations 2007.
|
|
|
Administration of Oestradiol and its ester-like derivatives
|
11(1) Notwithstanding Regulation 10 (1), a registered veterinary practitioner may, in accordance with an animal remedies authorisation, administer an authorised animal remedy containing oestradiol 17β or its ester-like derivatives only for:
|
| |
(a) treatment of foetus maceration or mummification in bovine animals,
|
| |
(b) treatment of pyometra in bovine animals.
|
| |
(2) A person, other than a registered veterinary practitioner, or a person licensed in accordance with Regulation 23 or 30 of the European Communities (Animal Remedies) Regulations 2007, at the premises referred to in the relevant licence, shall not possess an authorised animal remedy covered by this Regulation.
|
|
|
Register of Animal Remedies administered
|
12. (1) A registered veterinary practitioner to whom Regulation 8, 9, 10 or 11 applies shall cause to be established and maintained a register of all authorised animal remedies administered under those Regulations by him or her or pursuant to a prescription issued by him or her.
|
| |
(2) A registered veterinary practitioner shall, at the time of administering or prescribing an animal remedy pursuant to Regulation 8, 9, 10 or 11, enter in the register maintained under this Regulation the following particulars, that is to say:
|
| |
(a) the nature of the treatment given to the farm animal concerned,
|
| |
(b) the animal remedy administered,
|
| |
(c) the date of the treatment,
|
| |
(d) the identity of the farm animal concerned and
|
| |
(e) the date of expiry of the withdrawal period referred to in Regulation 41(5)(b)(ii) or (iii) of the European Communities (Animal Remedies) Regulations 2007.
|
| |
(3) If a person other than a registered veterinary practitioner administers, pursuant to Regulation 8, 9 or 10 , an authorised animal remedy to a farm animal he or she shall on that day inform the registered veterinary practitioner who issued the prescription in respect of the animal remedy of the fact and the registered veterinary practitioner shall thereupon enter in the register maintained under this Regulation the particulars specified in paragraph (2).
|
| |
(4) If a person other than a registered veterinary practitioner administers, pursuant to Regulation 8, 9 or 10, an authorised animal remedy to a farm animal the registered veterinary practitioner shall, in addition to entering the particulars specified in paragraph (2), in the register maintained under this Regulation, enter therein the date of issue by him or her of the relevant prescription.
|
| |
(5) A registered veterinary practitioner shall maintain an entry in a register maintained under this Regulation for a period of 5 years and shall make it available for inspection by an authorised officer on a request being made in that behalf by the officer.
|
|
|
Prohibition on administration of Authorised Animal Remedy
|
13. Notwithstanding Regulations 8, 9, 10 or 11, a person shall not administer or cause to be administered an authorised animal remedy, to which any of those Regulations apply, to -
|
| |
(a) a farm animal intended for fattening,
|
| |
(b) a castrate farm animal other than an equid, or
|
| |
(c) a reproductive farm animal during the fattening period at the end of its breeding life.
|
|
|
Laboratories
|
14. (1) The Minister may, for the purposes of these Regulations and the Council Directives, designate, by instrument in writing, a laboratory as an approved laboratory, and the persons for the time being employed or engaged in the analysis of specimens at a laboratory so designated may perform the functions of an approved laboratory specified in these Regulations and the Council Directives.
|
| |
(2) The Minister may, in accordance with Article 14 of Council Directive 96/23/EC of 29 April 1996, designate, in the plan submitted by him or her under Article 5 of that Directive, a laboratory as a national reference laboratory, and the persons for the time being employed or engaged in the analysis of specimens at a laboratory so designated may perform the functions of a national reference laboratory specified in these Regulations and the Council Directives.
|
|
|
Restriction of Movement of Certain Animals
|
15. (1) If -
|
| |
(a) an authorised officer has reasonable grounds for believing that in relation to a farm animal, meat, meat product, other food of animal origin or an animal remedy there has been a contravention of these Regulations.
|
| |
(b) an authorised officer receives a notification in writing from an approved laboratory or a national reference laboratory, that a specimen taken from a farm animal, meat, meat product or other food of animal origin was found, on analysis at the laboratory concerned -
|
| |
(i) to contain a prohibited animal remedy or a substance to which paragraph (1) of Regulation 3 applies, or
|
| |
(ii) to contain a residue of an authorised animal remedy in excess of the maximum residue limit in respect thereof,
|
| |
(c) a prohibited animal remedy or a substance to which paragraph (1) of Regulation 3 applies is found on land or premises, or
|
| |
(d) any plant, machinery, instrument, cartridge, container, utensil, label, package, insert or other thing to which paragraph (5) of Regulation 3 applies is found on land or premises,
|
| |
the authorised officer may give a direction, in accordance with paragraph (2), to -
|
| |
(i) the owner or occupier of -
|
| |
(I) the land or premises on which the authorised officer concerned has reasonable grounds for believing that the contravention referred to in paragraph (a) has taken place,
|
| |
(II) the land or premises on which the specimen referred to in paragraph (b) was taken, or
|
| |
(III) the land or premises to which paragraph (c) or (d) applies,
|
| |
as may be appropriate, or
|
| |
(ii) the owner or person in charge or control of -
|
| |
(I) the farm animal, meat, meat product or other food of animal origin to which paragraph (a) or (b) applies, or
|
| |
(II) any farm animal, meat, meat product or other food of animal origin found on land or premises to which paragraph (c) or (d) applies,
|
| |
as may be appropriate.
|
| |
(2) A direction under paragraph (1) shall be in writing and may, subject to paragraph (5) -
|
| |
(a) prohibit the movement of-
|
| |
(i) all farm animals, meat, meat products, or other food of animal origin, or
|
| |
(ii) such farm animal, meat, meat product or other food of animal origin as is specified in the direction,
|
| |
from such land or premises or any part thereof as is specified in the direction,
|
| |
(b) prohibit the movement of all farm animals, meat, meat products or other food of animal origin into such land or premises,
|
| |
(c) prohibit the sale or slaughter of such farm animals as may be specified in the direction, or
|
| |
(d) require the owner or occupier of such land or premises or the person in charge of or in control of the farm animal, meat, meat product or food concerned to comply with such other restrictions relating to the movement of farm animals, meat, meat products or food of animal origin as may be specified in the direction for such period as may be specified in the direction.
|
| |
(3) An authorised officer may by direction in waiting amend or revoke a direction given by him or her under this Regulation, including a direction under this paragraph.
|
| |
(4) A direction under this Regulation shall remain in force until it is revoked.
|
| |
(5) An authorised officer may, on the application in writing of the owner of a farm animal to which a direction under this Regulation applies, and on the taking from such farm animal by an authorised officer and analysis at an approved laboratory of such specimens (if any) as the authorised officer considers appropriate, issue a permit allowing the movement of the farm animal concerned into or out of the land or premises or part thereof to which the direction relates, or the sale or slaughter of such farm animal.
|
| |
(6) The costs of the taking and analysis of a specimen pursuant to paragraph (5) shall be borne by the owner of the farm animal concerned and shall be recoverable by the Minister-
|
| |
(a) in any court of competent jurisdiction as a simple contract debt, or
|
| |
(b) by deducting the costs from any sum due or becoming due from the Minister to the owner.
|
|
|
Testing of Animals if Illegal Treatment established
|
16. (1) If an authorised officer receives a notification in writing from an approved laboratory or a national reference laboratory, that on analysis at the laboratory concerned, a specimen taken by him or her from a farm animal was found to contain a prohibited animal remedy or a substance to which Regulation 3(1) applies, an authorised officer shall, in accordance with Article 17 of Council Directive 96/23/EC of 29 April 1996, take specimens from a statistically representative sample of the batch of animals to which the said farm animal belongs.
|
| |
(2) If, in relation to not less than half of the specimens taken under paragraph (1), an authorised officer receives a notification in writing from an approved laboratory or a national reference laboratory, that the specimens were found, on analysis at the laboratory concerned, to contain a prohibited animal remedy or a substance to which Regulation 3(1) applies -
|
| |
(a) a direction served under Regulation 15 in respect of those farm animals or in respect of the land or premises at which they are kept shall remain in force for a period of not less than 12 months, and
|
| |
(b) specimens shall be taken by an authorised officer from all farm animals in the batch referred to in paragraph (1) other than those farm animals from which specimens have been taken under that paragraph.
|
| |
(3) The costs of the taking and analysis of a specimen under this Regulation shall be borne by the owner or person in charge of the farm animal concerned and shall be recoverable by the Minister-
|
| |
(a) in any court of competent jurisdiction as a simple contract debt, or
|
| |
(b) by deducting the costs from any sum due or becoming due from the Minister to the owner.
|
| |
(4) Paragraph (2)(b) does not apply where the owner or person in charge of the farm animal concerned consents to their destruction in accordance with Regulation 19.
|
|
|
Surrender of Identity Cards
|
17. (1) Any person having in his or her possession, or under his or her control -
|
| |
(a) an identity card issued in respect of an animal on land or premises to which a direction under Regulation 15 applies, or
|
| |
(b) an identity card issued in respect of an animal to which a direction under the said Regulation applies,
|
| |
shall surrender the identity card to an authorised officer.
|
| |
(2) The owner or occupier of land or premises to which a direction under Regulation 15 applies shall make a declaration in writing in the form specified in the direction of the number and species of animals on the land or premises.
|
| |
(3) The owner or person in charge or control of an animal to whom a direction under Regulation 15 is addressed shall make a declaration in writing in the form specified in the direction of all land or premises of which he or she is the owner or occupier and the number and species of animals on such land or premises.
|
| |
(4) If the owner or person in charge of an animal referred to in paragraph (1) or the owner or occupier of land on which such animal is for the time being kept does not have in his or her possession or under his or her control the identity card in respect of the animal concerned then, he or she shall inform an authorised officer of the place at which, and the name and address of the person from whom, it may be obtained.
|
|
|
Marking of Animals
|
18. (1) If an authorised officer receives a notification in writing from an approved laboratory or a national reference laboratory, that a specimen taken from a farm animal was found, on analysis at the laboratory concerned, to contain a prohibited animal remedy or a substance to which Regulation 3(1) applies, the official mark inindelible form, shall be affixed to the farm animal, from which the specimen was taken, in such manner as an authorised officer considers appropriate.
|
| |
(2) If -
|
| |
(a) an authorised officer has reasonable grounds for believing that there is present in a farm animal a residue of an authorised animal remedy exceeding the maximum residue limit,
|
| |
(b) a specimen taken from a farm animal is shown, on analysis at an approved laboratory, to contain a residue of an authorised animal remedy exceeding the maximum residue limit,
|
| |
(c) an authorised officer has reasonable grounds for believing that a prohibited animal remedy or a substance to which Regulation 3(1) applies has been administered to a farm animal,
|
| |
the official mark (other than in indelible form) shall be affixed to the farm animal concerned in such manner as an authorised officer considers appropriate.
|
| |
(3) It shall not be lawful for a person, other than a person specified hereunder, to have in his or her possession or under his or her control a farm animal to which a mark has been affixed pursuant to this Regulation, that is to say:
|
| |
(a) a person to whom a direction under Regulation 15 is addressed,
|
| |
(b) an authorised officer, or
|
| |
(c) a person authorised in that behalf by an authorised officer
|
| |
(4) A person shall not remove, or attempt to remove or obscure other than in accordance with the instructions of an authorised officer, the official mark affixed under this Regulation.
|
| |
(5) A person shall not move an animal to which the official mark is affixed from the land or premises at which it was affixed other than for the purpose of destroying such animal in accordance with Regulation 19.
|
| |
(6) A person shall not sell or slaughter an animal to which the official mark is affixed.
|
|
|
Destruction of Animal where Illegal Treatment established
|
19. (1) If the official mark has been applied to a farm animal pursuant to Regulation 18(1) or if an authorised officer receives a notification in writing from an approved laboratory or a national reference laboratory, as the case may be, that a specimen taken from a farm animal was found, on analysis at the laboratory concerned, to contain a prohibited animal remedy or a substance to which Regulation 3 (1) applies, the Minister shall destroy or cause to be destroyed the farm animal concerned and its carcase in such manner as he or she deems appropriate.
|
| |
(2) Notwithstanding Regulations 4 and 5, a person may have in his or her possession or under his or her control an animal or carcase of an animal to which either of those Regulations apply for the purpose of complying with paragraph (1).
|
| |
(3) The cost of destroying a farm animal or its carcase under this Regulation shall, subject to paragraph (4), be borne by the owner of the animal and may be recoverable by the Minister-
|
| |
(a) in any court of competent jurisdiction as a simple contract debt, or
|
| |
(b) by deducting the costs from any sum due or becoming due from the Minister to the owner.
|
| |
(4) If the owner and person in charge of an animal destroyed under this Regulation are not the same person both the owner and the person in charge of the animal shall be jointly and severally liable for the costs of the destruction of the animal and its carcase.
|
|
|
Lawful to sell etc. Animal where Withdrawal Period has elapsed
|
20. (1) A person may export or sell a farm animal to which an animal remedy has been administered in accordance with Regulation 8, 9 10 or 11, if the withdrawal period in respect thereof pursuant to Council Regulation (EEC) No. 2377/90 of 26 June 1990 has elapsed, there has been compliance with Regulation 43(2) and (3) of the European Communities (Animal Remedies) Regulations 2007 and the Animal Remedies Record is available for inspection on request by an authorised officer.
|
| |
(2) A person may export or sell meat, a meat product or other food derived from a farm animal to which an animal remedy has been administered in accordance with Regulation 8, 9 10 or 11, or to process such meat, meat product or other food if the withdrawal period in respect of the animal remedy concerned set out in Council Regulation (EEC) No. 2377/90 of 26 June 1990 had elapsed at the time the farm animal concerned was slaughtered and there had in respect of that farm animal been compliance with Regulation 43(2) and (3) of the European Communities (Animal Remedies) Regulations 2007, and the Animal Remedies Record in respect of that farm animal is available for inspection on request by an authorised officer.
|
| |
(3) A person may slaughter an animal, to which an animal remedy has been administered in accordance with Regulation 8, 9 10 or 11, if the withdrawal period in respect thereof set out in Council Regulation (EEC) No. 2377/90 of 26 June 1990 has elapsed and there has been compliance with Regulation 43(2) and (3) of the European Communities (Animal Remedies) Regulations 2007, and the Animal Remedies Record is available for inspection on request by an authorised officer.
|
| |
(4) Notwithstanding paragraph (1) or (2), a person may import, export or sell a high-value horse, in particular, a racehorse, competition horse, circus horse or horse intended for stud purposes or for exhibition purposes, including a registered equid, to which an animal remedy consisting of or containing -
|
| |
(a) allyl trenbolone has been administered in accordance with Regulation 8, or
|
| |
(b) a beta-agonist has been administered in accordance with Regulation 9, before the end of the withdrawal period in respect thereof provided that the certificate or passport in respect of the animal concerned specifies the nature, method and date of administration of the animal remedy.
|
|
|
Register of Certain Substances etc
|
21. (1) A person who engages in -
|
| |
(a) the manufacture of a substance to which Regulation 3 applies, or
|
| |
(b) the importation, purchase or sale of such substance,
|
| |
shall cause to be established and maintained a register containing the particulars specified in paragraph (2).
|
| |
(2) A person to whom paragraph (1) applies shall enter in chronological order the following particulars in the register established and maintained by him or her under this Regulation:
|
| |
(a) the quantities of each substance referred to in paragraph (1) manufactured, imported, purchased or otherwise acquired by him or her;
|
| |
(b) the quantities of each such substance sold for or used in the manufacture of pharmaceutical or veterinary medicinal products; and
|
| |
(c) the name of the person to whom such quantities were sold or from whom they were purchased or otherwise acquired and the address at which he or she ordinarily resides.
|
| |
(3) A person to whom this Regulation applies shall furnish the Minister with such particulars required to be entered in a register under this Regulation as the Minister may from time to time direct in such form as he or she may direct.
|
|
|
Detection by Processors of Illegal Treatment
|
22. (1) The owner or person in charge of an approved establishment shall, not later than the 31st day of October in each year prepare and submit to the Minister a plan for the detection, in the year immediately following the year in which the plan is prepared and submitted, of substances, veterinary drugs and contaminants specified in Annex 1 to Council Directive 96/23/EC of 29 April 1996 in -
|
| |
(a) farm animals presented for slaughter, and
|
| |
(b) meat, meat products or other food derived from such animals,
|
| |
at the establishment, and the plan shall, if approved by the Minister, be carried out by the owner or person in charge of the establishment in accordance with its terms.
|
| |
(2) The owner or person in charge of an approved milk processing plant shall, not later than the 31st day of October in each year prepare and submit to the Minister a plan for the detection, in the year immediately following the year in which the plan is prepared and submitted, of substances, veterinary drugs and contaminants specified in the Annex referred to in paragraph (1) in milk presented for processing at the milk processing plant, and the plan shall, if approved by the Minister, be carried out by the owner or person in charge of the milk processing plant in accordance with its terms.
|
| |
(3) Without prejudice to the generality of paragraphs (1) and (2), the Minister may direct that a plan prepared and submitted under this Regulation shall contain such provisions and comply with such requirements as are specified in the direction, including provisions and requirements relating to -
|
| |
(a) the form of the plan,
|
| |
(b) the taking of specimens at an approved establishment or an approved milk processing plant, as may be appropriate.
|
| |
(c) the manner in which the analysis of specimens is to be carried out,
|
| |
(d) the frequency with which testing for illegal treatment is to be conducted,
|
| |
(e) the number, species and age of animals to be tested for illegal treatment,
|
| |
(f) the foods of animal origin to be tested for illegal treatment, and
|
| |
(g) measures to be taken by the owner or person in charge of the establishment or milk processing plant concerned if illegal treatment is detected.
|
| |
(4) A plan prepared and submitted under this Regulation shall comply with a direction given under paragraph (3).
|
| |
(5) The Minister may approve a plan prepared and submitted under this Regulation.
|
| |
(6) The Minister may within a period of 60 days of the submission of a plan to him or her under this Regulation require, by notice in writing, that the plan concerned be modified in such manner as he or she directs.
|
| |
(7) If the Minister requires that a plan submitted under paragraph (1) or (2) be modified the owner or person in charge of the establishment or milk processing plant concerned shall modify the plan in accordance with directions of the Minister and shall within a period of 30 days of receipt by him or her of a notice under paragraph (6) submit the plan as so modified to the Minister for approval by him or her.
|
| |
(8) If the Minister does not, within a period of 60 days of the submission of a plan to him or her under paragraph (1) or (2)-
|
| |
(a) approve,
|
| |
(b) refuse approval of, or
|
| |
(c) require under paragraph (7) the modification of,
|
| |
the plan concerned, it, for the purposes of this Regulation, is deemed to have been approved by him or her.
|
| |
(9) The owner or person in charge of an establishment or approved milk processing plant shall, not later than the 31st day of March in each year, prepare and submit to the Minister a report, on the implementation in the immediately preceding year, of a plan approved by the Minister under this Regulation.
|
| |
(10) Without prejudice to the generality of paragraph (9), a report submitted under that paragraph shall in respect of the year to which the report relates include the following, that is to say:
|
| |
(a) in the case of an establishment, the number and species of animals slaughtered at the approved establishment concerned,
|
| |
(b) the name of the person responsible for ensuring the implementation of the plan at the establishment or the approved milk processing plant concerned,
|
| |
(c) in the case of an establishment, the number and species of animals from which specimens were taken and analysed under the plan,
|
| |
(d) in the case of a milk processing plant, particulars of the specimens analysed under the plan;
|
| |
(e) in the case of an establishment, the number and species of animals from which specimens taken were found on analysis to contain -
|
| |
(i) a prohibited animal remedy or a substance to which Regulation 3(1) applies, or
|
| |
(ii) a residue of an authorised substance in excess of the maximum residue limit,
|
| |
(f) particulars in relation to food of animal origin from which specimens taken were found on analysis to contain -
|
| |
(i) a prohibited animal remedy or a substance to which Regulation 3(1) applies, or
|
| |
(ii) a residue of an authorised substance in excess of the maximum residue limit,
|
| |
(g) the name of the person from whom each animal to which subparagraph (e) applies, or food of animal origin to which subparagraph (f) applies, was purchased, and the address at which he or she ordinarily resides,
|
| |
(h) in the case of an establishment, the name of the person from whom each animal (from which a food of animal origin to which subparagraph (f) applies, was obtained) was purchased, and the address at which he or she ordinarily resides, and
|
| |
(i) particulars of measures taken in respect of each animal to which subparagraph (e) applies, or food of animal origin to which subparagraph (f) applies.
|
| |
(11) The Minister may by direction in writing, -
|
| |
(a) require the owner or person in charge of an establishment to take such measures for the detection of substances, veterinary drugs and contaminants specified in the Annex referred to in paragraph (1), in farm animals presented for slaughter at the establishment concerned, as are specified in the direction.
|
| |
(b) require the owner or person in charge of a milk processing plant to take such measures for the detection of substances, veterinary drugs and contaminants specified in the Annex referred to in paragraph (1), in milk presented for processing at the milk processing plant.
|
| |
(12) If, in the carrying out of a plan under this Regulation, or the taking of measures pursuant to a direction under paragraph (11), a specimen taken from an animal or food of animal origin is found, on analysis, to contain a substance to which Regulation 3(1) applies, a prohibited animal remedy or an amount of an authorised animal remedy in excess of the maximum residue limit, the owner or person in charge of the approved establishment, approved milk processing plant or abattoir, as the case may be, at which such specimen was taken shall immediately inform the Minister or an authorised officer of that finding and of the name and address of the person who presented the animal concerned for slaughter, or food of animal origin concerned for processing, at such approved establishment, approved milk processing plant or abattoir.
|
| |
(13) In this Regulation -
|
| |
“approved establishment” means an establishment within the meaning of the European Communities (Food and Feed Hygiene) Regulations 2005 (S.I.No. 910 of 2005) used for the slaughter of animals;
|
| |
“milk processing plant” means a milk treatment establishment, a milk processing establishment or a milk processing establishment with limited production capacity, approved in accordance with the European Communities (Food and Feed Hygiene) Regulations, 1995 (S.I. No. 910 of 2005).
|
|
|
Evidence by certificate in proceedings for an offence
|
23. (1) In proceedings for an offence consisting of a contravention of these Regulations, a certificate purporting to be signed by a person employed at an approved laboratory or a national reference laboratory, stating the capacity in which that person is so employed and stating any one or more of the following, namely -
|
| |
(a) that the person received a specimen submitted to the approved laboratory or the national reference laboratory,
|
| |
(b) that, for such period as is specified in the certificate, the person had in his or her custody a specimen so submitted,
|
| |
(c) that the person gave to such other person as is specified in the certificate a specimen so submitted, or
|
| |
(d) that the person carried out any procedure for the purpose of detecting the presence, in a specimen so submitted, of an animal remedy, or that the specimen concerned contained such animal remedy or such amount thereof as is specified in the certificate,
|
| |
shall, unless the contrary is proved, be evidence of the matters stated in the certificate.
|
| |
(2) In proceedings for an offence under these Regulations the court may, if it considers that the interests of justice so require, direct that oral evidence of the matters stated in a certificate under this Regulation be given, and the court may for the purpose of receiving oral evidence adjourn the proceedings to a later date.
|
|
|
Forgery of Documents
|
24. (1) A person shall not forge or utter knowing it to be forged -
|
| |
(a) a register purporting to be established and maintained under these Regulations or a document purporting to be an extract therefrom (hereafter in this Regulation referred to as “a forged register”), or
|
| |
(b) a direction, permit or other document purporting to be issued, granted or given under these Regulations (hereafter in this Regulation referred to as “a forged document”).
|
| |
(2) It shall not be lawful for a person to alter with intent to defraud or deceive, or to utter knowing it to be so altered -
|
| |
(a) a register established and maintained under these Regulations or an extract therefrom (hereafter in this Regulation referred to as “an altered register”), or
|
| |
(b) a direction, permit or other document issued, granted or given under these Regulations (hereafter in this Regulation referred to as “an altered document”),
|
| |
(3) A person shall not have, without lawful authority, in his or her possession a forged register, forged document, altered register or altered document.
|
|
|
Service of Documents
|
25. A direction or other document under these Regulations shall be addressed to the person concerned by name, and may be served on or given to the person in one of the following ways:
|
| |
(a) by delivering it to the person,
|
| |
(b) by leaving it at the address at which the person ordinarily resides or, in a case in which an address for service has been furnished, at that address.
|
|
|
Evidential Burden
|
26. In proceedings for an offence consisting of a contravention of Regulation 3(2), 5, or 6(1) or (4), it is not necessary to negative by evidence the existence of an animal remedies authorisation, a manufacturer's licence, a licence under Regulation 17 of the European Communities (Animal Remedies) Regulations 2007 or a product authorisation under the Medical Preparations (Licensing and Sale) Regulations, 1996 (S.I. No. 43 of 1996), and accordingly the onus of proving the grant or issue of such authorisation or licence is on the defendant.
|
|
|
Persons not entitled to Community Aid
|
27. (1) If the owner or person for the time being in charge of an establishment is convicted of an offence consisting of a contravention of Regulation 22(9), (10)(e), (f), (g) or (h), or (12), or 24, or section 16 of the Act, none of the following, that is to say:
|
| |
(a) the owner,
|
| |
(b) a company in which he or she has a controlling interest, or
|
| |
(c) in circumstances where the owner is a company, a related company,
|
| |
is entitled to receive Community aid -
|
| |
(i) for a period of 12 months commencing on the date of such conviction, or
|
| |
(ii) that but for the making of this Regulation would be payable in respect of the whole or part of such period.
|
| |
(2) In this Regulation -
|
| |
“company” has the same meaning as it has in section 2 of the Companies Act, 1963 (No. 33 of 1963);
|
| |
“holding company” means a holding company within the meaning of the section 155;
|
| |
“related company” in relation to a company, means the holding company or a subsidiary of that company, or a company that is a subsidiary of the first-mentioned company's holding company;
|
| |
“subsidiary” means a subsidiary within the meaning of the said section 155;
|
| |
(3) In this Regulation, a person has a controlling interest in a company if circumstances exist whereby, were that person a company, the first-mentioned company would be that company's subsidiary.
|
|
|
Implied Condition in Contract of Sale
|
28(1) In every contract of sale there shall be an implied condition on the part of the seller that all reasonable precautions have been taken and all due diligence has been exercised to ensure—
|
| |
( a ) in the case of the sale of an animal, that the animal was not treated with any animal remedy and, in the case of an agreement to sell an animal, the animal was not so treated and will not be so treated prior to the time when the property is to pass, and
|
| |
( b ) in the case of the sale of, or an agreement to sell, the carcase of any animal or food derived from any animal, that the animal had not been treated with any animal remedy,
|
| |
otherwise than in accordance with these Regulations.
|
| |
( 2 ) ( a ) Subject to sub-paragraph (b), any term of a contract implied by virtue of paragraph (1) may be negatived or varied, by an express term in the contract, in so far as the first-mentioned term relates to an animal remedy—
|
| |
(i) which has been administered to an animal before being imported into the State, and
|
| |
(ii) (ii) in respect of which the Minister has granted an exemption from destruction,
|
| |
but only if the express term is fair and reasonable and has been specifically brought to the attention of the buyer.
|
| |
( b ) Sub-paragraph (a) does not apply to an animal imported into the State where the sale of the animal would not be in accordance with a condition imposed by the Minister on the exemption.
|
| |
(3) Any term of a contract implied by virtue of paragraph (1) may not be negatived or varied in so far as it relates to an animal remedy other than in respect of an animal remedy to which paragraph (2) relates.
|
|
|
Inspection by authorised officers, etc.
|
29(1) If an authorised officer or member of the Garda Síochána or an officer of Customs and Excise has reasonable cause to suspect that—
|
| |
( a ) the manufacture, importation, preparation, handling, storage, transport, exportation, distribution, sale, supply or use of an animal remedy or any ingredient for an animal remedy is taking place or has taken place in, on, under or from any land, premises or in, on or from any vehicle,
|
| |
( b ) an offence is being or has been committed under these Regulations in, on, under or from any land, premises or in, on or from any vehicle,
|
| |
( c ) any land or premises is used for or in connection with the breeding, rearing, fattening, keeping, exhibiting, selling or transporting of animals,
|
| |
( d ) any land or premises is an establishment or is used for or in connection with the slaughter of animals,
|
| |
( e ) in, on, under or from any land or premises or in, on or from any vehicle, there is or was any animal of any species to which an animal remedy is being or has been administered or there is or was any food derived from such an animal or any carcase of such an animal, or
|
| |
( f ) in, on, under or from any land or premises or in, on or from any vehicle, there is or was any animal remedy, or any ingredients for animal remedies, or any machinery, instruments or other thing used in the manufacture, preparation, handling, storage, transport, exportation, distribution, sale, supply or use of animal remedies or ingredients for animal remedies,
|
| |
the authorised officer or member of the Garda Síochána or officer of Customs and Excise (in this section referred to as “the relevant person”) may, subject to paragraph (2), stop any such vehicle or enter (if necessary by force) any such land or premises, or land or premises used in connection with such land or premises, or any such vehicle, and there, or at any other place, and with such authorised officers, members of the Garda Siochána and officers of Customs and Excise (if any) as the relevant person considers appropriate—
|
| |
(i) search for and examine, inspect or test any animals, food derived from animals or carcases of animals or anything believed to be an animal remedy or an ingredient for an animal remedy or anything to which subparagraph (f) relates,
|
| |
(ii) take such specimens (including blood, urine, faeces, tissue or remains of implants) from any animals, food derived from animals or carcases of animals, and may for that purpose perform or cause to be performed any procedure (including surgery) as is considered necessary on such animals, food or carcases,
|
| |
(iii) take such reasonable samples of or from any substances or of or from a thing which may be considered appropriate for the purposes of these Regulations,
|
| |
(iv) seize and detain anything to which subparagraph (f) relates or anything which is believed to be or to contain an animal remedy or an ingredient for an animal remedy kept, used or intended to be used in contravention of the provisions of these Regulations,
|
| |
(v) search for and examine any document and take extracts from and copies of any such document,
|
| |
(vi) seize and detain an animal—
|
| |
(I) in respect of which it is, with reasonable grounds, believed by the relevant person that a prohibited animal remedy or ingredient for an animal remedy has been administered to it in contravention of these Regulations, and
|
| |
(II) in relation to which either or both—
|
| |
(A) the relevant person is aware that an application has been made or will be made for the destruction of the animal, and
|
| |
(B) the relevant person has reasonable grounds for believing that the animal has been or may be moved in contravention of any notice duly served,
|
| |
in accordance with these Regulations,
|
| |
(vii) require any person who is suspected to be, or to have been engaged in the manufacture, preparation, handling, storage, transport, exportation, distribution, sale, supply or use of, or any person who is suspected to have possession or control of or to have kept or to keep, any animal remedy, ingredient for an animal remedy, animal, food derived from animals, carcases of animals or anything to which subparagraph (f) relates, or any person who is suspected to be, or to have been, engaged in the breeding, rearing, fattening, keeping, exhibiting, selling or transporting or in the possession or control of any animal—
|
| |
(I) in the case of any documents in the possession or control of that person or any such remedy, ingredient, animal, food, carcase or thing, to produce them to the relevant person or any authorised officer, member of the Garda Síochána or officer of Customs and Excise,
|
| |
(II) in the case of any information in relation to such document, remedy, animal, food, carcase or thing which may be required (including the source of that document, remedy, animal, food, carcase or thing), to furnish them to the relevant person or any authorised officer, member of the Garda Síochána or officer of Customs and Excise,
|
| |
(viii) require any person, being the owner or the person in charge of animals, or the owner or occupier of, or employed in or on, lands or premises so entered to give assistance, to carry out such instructions and to give such information as may be reasonably necessary for the purposes of subsubparagraphs (i) to (vii), and
|
| |
(ix) require any person who is for the time being in charge or control of any vehicle so stopped or entered—
|
| |
(I) to refrain from moving it, and
|
| |
(II) to give assistance, to carry out such instructions and to give such information as may be reasonably necessary for the purposes of subsubparagraphs (i) to (vii).
|
| |
(2) The functions of a relevant person under this Regulation may only be exercised in respect of a dwelling or so much of a vehicle or premises as constitutes a dwelling where the relevant person has reasonable cause to suspect that, before a search warrant could be sought in relation to the dwelling under Regulation 55, anything to which the said paragraph (1) relates—
|
| |
( a ) is being destroyed or disposed, or
|
| |
( b ) is likely to be destroyed or disposed.
|
| |
(3) An authorised officer, member of the Garda Síochána or officer of Customs and Excise accompanying the relevant person may exercise all the functions conferred on the relevant person by virtue of paragraph (1) or (2).
|
|
|
Search warrant
|
30(1) If a judge of the District Court is satisfied by information on oath of an authorised officer, member of the Garda Síochána or an officer of Customs and Excise that there is reasonable cause for suspecting that—
|
| |
( a ) evidence of or relating to the commission or intended commission of an offence under these Regulations is to be found in, on or under any land or premises or in or on any vehicle and that such land, premises or vehicle or any part thereof consists of a dwelling, or
|
| |
( b ) there is or was or is intended to be in, on or under any land or premises, in or on any vehicle and that such land, premises or vehicle or any part thereof consists of a dwelling, any animal remedy or ingredient for an animal remedy in relation to which a contravention of these Regulations, is being or has been or is intended to be committed, or
|
| |
( c ) a document directly or indirectly relating to, or connected with, a transaction or dealing which was, or an intended transaction or dealing which would if carried out be, an offence under these Regulations, is in the possession or under the control of a person in, on or under any land or premises or in or on any vehicle and that such land, premises or vehicle or any part thereof consists of a dwelling,
|
| |
such judge may issue a search warrant under this Regulation.
|
| |
(2) A search warrant issued under this Regulation shall be expressed and operate to authorise a named authorised officer, named member of the Garda Síochána or named officer of Customs and Excise, accompanied by such authorised officers, members of the Garda Síochána and officers of Customs and Excise as the named officer or member thinks necessary, at any time or times within one month from the date of issue of the warrant, on production if so requested of the warrant to enter (if necessary by force) the land, premises or vehicle named in the warrant.
|
| |
(3) Where any premises, land or vehicle is entered pursuant to a warrant issued under this section, an authorised officer, a member of the Garda Síochána or an officer of Customs and Excise so entering may—
|
| |
( a ) stop and detain any person found in, on or under such land or premises, or in or on such vehicle, for the purpose of searching that person and to search or cause to be searched that person, and
|
| |
( b ) exercise all or any of the powers referred to in Regulation 29.
|
|
|
Search of suspects, etc.
|
31(1) If with reasonable cause a member of the Garda Siochána or an officer of Customs and Excise suspects that a person is in possession in contravention of these Regulations of an animal remedy or an ingredient for an animal remedy, the member or officer may without warrant—
|
| |
( a ) search or cause to be searched by such a member or officer the person and, if the member or officer considers it necessary for that purpose, detain the person for such time as is reasonably necessary to carry out the search,
|
| |
( b ) search or cause to be searched by such a member or officer any vehicle in which the member or officer suspects that such substance may be found and for the purpose of carrying out the search, if any such member or officer thinks fit, require the person who is, for the time being, in charge or control of the vehicle to bring it to a stop and when stopped to refrain from moving it or, in case the vehicle is already stationary, to refrain from moving it, or
|
| |
( c ) seize and detain, or cause to be seized and detained by such a member or officer, anything found in the course of a search under this section which any such member or officer reasonably suspects to be something which might be required as evidence in proceedings for an offence under these Regulations.
|
| |
(2) Where a member of the Garda Síochána or an officer of Customs and Excise (as the case may be) decides to search or cause to be searched a person under this section the member or officer may require the person to accompany that member or officer to either a Garda Síochána station or a customs office for the purpose of being so searched at that station or office.
|
| |
(3) A person who fails to comply with a requirement under this Regulation shall be guilty of an offence.
|
|
|
Power of arrest.
|
32(1) Where with reasonable cause a member of the Garda Síochána suspects that—
|
| |
( a ) an offence under these Regulations has been committed and so suspects a person of having committed the offence, or
|
| |
( b ) a person is committing or has committed an offence under these Regulations in relation to the manufacture, importation, preparation, handling, storage, transport, exportation, distribution, sale, supply or use of any animal remedy or any ingredient for an animal remedy, the possession of which by such a person would be prohibited by these Regulations,
|
| |
the member may arrest the person without warrant.
|
| |
(2) Where with reasonable cause a member of the Garda Síochána—
|
| |
( a ) suspects that an offence under these Regulations has been committed or attempted, and
|
| |
( b ) suspects a person of having committed the offence or having made the attempt,
|
| |
then the member may arrest the person without warrant if—
|
| |
(i) with reasonable cause the member suspects that the person, unless arrested, either will abscond for the purposes of evading justice or will obstruct the course of justice, or
|
| |
(ii) having enquired of the person, the member has reasonable doubts as to the person's identity or place of abode, or
|
| |
(iii) having enquired of the person, the member knows that the person does not ordinarily reside in the State, or has reasonable doubts as to whether the person so resides.
|
| |
(3) Nothing in sub-paragraphs (i), (ii) and (iii) of paragraph (2) shall apply where a person is required to accompany a member of the Garda Síochána or an officer of Customs and Excise to a Garda Síochána station or a customs office for the purpose of Regulation 31 and who fails to comply with the requirement.
|
|
|
Saving for certain power.
|
33. Nothing in these Regulations shall operate to prejudice any power to search, or to seize or detain property, which may, apart from these Regulations, be exercised by a member of the Garda Síochána or an officer of Customs and Excise.
|
|
|
Obstruction.
|
34. A person shall not obstruct or impede an authorised officer, member of the Garda Síochána or officer of Customs and Excise in the due exercise of any of the functions conferred or exercisable by the authorised officer, member of the Garda Síochána or officer of Customs and Excise under these Regulations.
|
|
|
Impersonation of authorised officer, etc. and possession of certain identity document.
|
35(1) A person who, with the intention to deceive—
|
| |
( a ) purports to be, or
|
| |
( b ) acts in a manner that would lead another person to believe that he or she is,
|
| |
a person duly appointed as an authorised person, officer, inspector, examiner or other officer of the Minister or a person otherwise duly appointed by or with the authority of the Minister or of any other purported member of the Government exercising any functions of the Minister either—
|
| |
(i) generally, or
|
| |
(ii) for the purposes of these Regulations,
|
| |
shall be guilty of an offence.
|
| |
(2) A person who, without lawful excuse, has in his or her possession any document which—
|
| |
( a ) has been,
|
| |
( b ) purports to be, or
|
| |
( c ) could lead another person to believe that it has been,
|
| |
duly issued for the purpose of identifying the person in possession of the document as a person duly authorised by, or a duly authorised officer, inspector, examiner or other officer of, the Minister or otherwise duly appointed by or with the authority of the Minister or, in the case of sub-paragraph (b) or (c), of any other purported member of the Government exercising any functions of the Minister either—
|
| |
(i) generally, or
|
| |
(ii) for the purposes of these Regulations,
|
| |
shall be guilty of an offence.
|
| |
(3) In this Regulation, references to the Minister include the Department of Agriculture and Food and any Minister of State at that Department and references to a purported member of the Government include any purported Department of State and any Minister of State at such Department.
|
|
|
Offences and penalties
|
36.(1) A person who -
|
| |
(a) contravenes Regulation 3,4,5, 6, 8, 9, 10, 11, 12, 13, 17, 18(3), (4), (5), (6), 20, 21, 22, 24,
|
| |
(b) fails to comply with a direction or requirement under Regulation 7(1)(a), 15 or 29, or
|
| |
(c) counsels, aids or abets a contravention of subparagraph (a) or (b),
|
| |
commits an offence and is liable on conviction to a fine not exceeding €5,000 or to a term of imprisonment not exceeding 6 months or to both.
|
| |
(2) An offence under these Regulations may be prosecuted by the Minister.
|
| |
(3) If an offence under these Regulations has been committed by a body corporate and it is proved to have been so committed with the consent or connivance of or to be attributable to any neglect on the part of any person who, when the offence was committed, was a director, manager, secretary or other officer of the body corporate, or a person purporting to act in any such capacity, that person, as well as the body corporate, commits an offence and is liable to be proceeded against and punished as if guilty of the first-mentioned offence.
|
| |
(4) If the affairs of a body corporate are managed by its members, paragraph (3) applies in relation to the acts and defaults of a member in connection with the functions of management as if the member is a director or manager of the body corporate.
|
|
|
Revocation and Saver
|
37. (1) The following are revoked-
|
| |
(a) the European Communities (Control of Oestrogenic, Androgenic, Gestagenic and Thyrostatic Substances) Regulations, 1988 (S.I. No. 218 of 1988.),
|
| |
(b) the European Communities (Control of Veterinary Medicinal Products and their Residues) Regulations 1990 (S.I. No. 171 of 1990),
|
| |
(c) the Control of Animal Remedies and their Residues Regulations 1998 (SI No. 507 of 1998).
|
| |
(2) A direction issued under Regulation 25 of the Control of Animal Remedies and their Residue Regulations 1998 is confirmed and may be dealt with as if given under the corresponding provision of these Regulations.
|
| |
(3) If paragraph (2) would conflict with the constitutional right of any person, the operation of that paragraph is subject to such limitation as is necessary to secure that it does not so conflict, but is otherwise of full force and effect.
|
| |
SCHEDULE
Official Mark
|
| | |
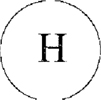
|
|
GIVEN under my Official Seal, this 2nd day of April 2007.
|
|

|
|
|
|
Mary Coughlan
Minister for Agriculture and Food.
|
|
| |
EXPLANATORY NOTE
|
| |
(This Note is not part of the instrument and does not purport to be a legal interpretation.)
|
| |
These Regulations implement in the State the provisions of Council Directive 96/22/EC of 29 April 1996 containing the prohibition in stockfarming of certain substances having hormonal or thyrostatic action and of beta agonists and Council Directive 96/23 of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products.
|
| |
1 O.J. L 125 of 23.5.96
2 O.J. L 125 of 23.5.96
3 OJ L 204. 11.8.2000
4 OJ L 224. 18.8.1990 |