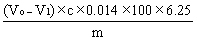S.I. No. 143/1994 - European Communities (Marketing of Feedingstuffs) (Amendment) Regulations, 1994.
S.I. No. 143 of 1994. | ||||||||||||||
EUROPEAN COMMUNITIES (MARKETING OF FEEDINGSTUFFS) (AMENDMENT) REGULATIONS, 1994. | ||||||||||||||
I, JOE WALSH, Minister for Agriculture, Food and Forestry, in exercise of the powers conferred on me by section 3 of the European Communities Act, 1972 (No. 27 of 1972), and for the purpose of giving effect to Commission Directive 93/28/EEC of 4 June 1993(1), hereby make the following Regulations: 1. (1) These Regulations may be cited as the European Communities (Marketing of Feedingstuffs) (Amendment) Regulations, 1994. | ||||||||||||||
(2) The European Communities (Marketing of Feedingstuffs) Regulations, 1984 to 1993 and these Regulations may be cited together as the European Communities (Marketing of Feedingstuffs) Regulations, 1984 to 1994. | ||||||||||||||
(3) The European Communities (Marketing of Feedingstuffs) Regulations, 1984 to 1993, and these Regulations shall be construed together as one. 2. The European Communities (Marketing of Feedingstuffs) Regulations, 1984 ( S.I. No. 200 of 1984 ) are hereby amended by the substitution, for paragraph 5.1 of Part 1 of the Third Schedule, of the following paragraph; | ||||||||||||||
5.1 DETERMINATION OF CRUDE PROTEIN. | ||||||||||||||
1. Purpose and Scope. | ||||||||||||||
To determine the crude protein content of feedingstuffs on the basis of the nitrogen content, determined according to the Kjeldahl method. | ||||||||||||||
2. Principle. | ||||||||||||||
The sample is digested by sulphuric acid in the presence of a catalyst. The acid solution is made alkaline with sodium hydroxide solution. The ammonia is distilled and collected in a measured quantity of sulphuric acid, the excess of which is titrated with a standard solution of sodium hydroxide. | ||||||||||||||
3. Reagents. | ||||||||||||||
3.1. Potassium sulphate. | ||||||||||||||
3.2. Catalyst: copper (II) oxide CuO or copper (II) sulphate pentahydrate, CuSO4·5H2O. | ||||||||||||||
3.3. Granulated zinc. | ||||||||||||||
3.4. Sulphuric acid, p20=1.84 g/ml. | ||||||||||||||
3.5. Sulphuric acid c(½H2SO4) = 0.5 mol/l. | ||||||||||||||
3.6. Sulphuric acid c(½H2SO4) = 0.1 mol/l. | ||||||||||||||
3.7. Methyl red indicator; dissolve 300 mg of methyl red in 100 ml of ethanol, σ = 95-96% (v/v). | ||||||||||||||
3.8 Sodium hydroxide solution (Technical grade may be used) ß = 40 g/100 ml (m/v:40%). | ||||||||||||||
3.9 Sodium hydroxide solution c = 0.25 ml/l. | ||||||||||||||
3.10 Sodium hydroxide solution c = 0.1 mol/l. | ||||||||||||||
3.11 Granulated pumice stone, washed in hydrochloric acid and ignited. | ||||||||||||||
3.12 Acetanilide(m.p.= 114°C,N = 10.36%). | ||||||||||||||
3.13 Sucrose (nitrogen free). | ||||||||||||||
4. Apparatus. | ||||||||||||||
Apparatus suitable for performing digestion, distillation and titration according to the Kjeldahl procedure. | ||||||||||||||
5. Procedure. | ||||||||||||||
5.1. Digestion. | ||||||||||||||
Weigh, to the nearest 0.001g, approximately 1g of sample and transfer the sample to the flask of the digestion apparatus. Add 15g of potassium sulphate (3.1.), an appropriate quantity of catalyst (3.2) (0.3 to 0.4g of copper (II) oxide or 0.9 to 1.2g of copper (II) sulphate pentahydrate), 25 ml of sulphuric acid (3.4) and a few granules of pumice stone (3.11) and mix. Heat the flask moderately at first, swirling from time to time if necessary until the mass has carbonized and the foam has disappeared; then heat more intensively until the liquid is boiling steadily. Heating is adequate if the boiling acid condenses on the wall of the flask. Prevent the sides from becoming overheated and organic particles from sticking to them. When the solution becomes clear and light green in colour continue to boil for another two hours (see observation 8.2). Turn off the heat and leave to cool. | ||||||||||||||
5.2 Distillation. | ||||||||||||||
Add carefully enough water to ensure complete dissolution of the sulphates. Allow to cool and then add a few granules of zinc (3.3). | ||||||||||||||
Place in the collecting flask of the distillation apparatus an exactly measured quantity of 25 ml of sulphuric acid (3.5) or (3.6) depending on the presumed nitrogen content. Add a few drops of methyl red indicator (3.7). | ||||||||||||||
Connect the digestion flask to the condenser of the distillation apparatus and immerse the end of the condenser in the liquid contained in the collecting flask to a depth of at least 1 cm (see observation 8.3). Slowly pour 100 ml of sodium hydroxide solution (3.8) into the digestion flask without loss of ammonia (see observation 8.1). | ||||||||||||||
Heat the flask until the distillation of ammonia is complete. | ||||||||||||||
5.3. Titration. | ||||||||||||||
Titrate the excess sulphuric acid in the collecting flask with sodium hydroxide solution (3.9) or (3.10) depending on the concentration of the sulphuric acid used in 5.2, until the end point is reached. | ||||||||||||||
5.4 Blank Test. | ||||||||||||||
To confirm that the reagents are free from nitrogen, carry out a blank test (digestion, distillation and titration) using 1g of sucrose (3.13) in place of the sample. | ||||||||||||||
6. Calculation of results. | ||||||||||||||
The content of crude protein is calculated according to the following formula: | ||||||||||||||
| ||||||||||||||
Where, | ||||||||||||||
| ||||||||||||||
7. Verification of the method. | ||||||||||||||
7.1 Repeatability. | ||||||||||||||
The difference between the results of two parallel determinations carried out on the same sample must not exceed: | ||||||||||||||
0.2% in absolute value, for crude protein contents of less than 20%; | ||||||||||||||
1.0% relative to the higher value, for crude protein contents from 20% to 40%; | ||||||||||||||
0.4% in absolute value, for crude protein contents of more than 40%. | ||||||||||||||
7.2 Accuracy. | ||||||||||||||
Carry out the analysis (digestion, distillation and titration) on 1.5 to 2.0g of acetanilide (3.12) in the presence of 1g of sucrose (3.13);1g acetanilide consumes 14.80 ml of sulphuric acid (3.5). Recovery must be at least 99%. | ||||||||||||||
8. Observations. | ||||||||||||||
8.1 Apparatus may be of the manual, semi-automatic or automatic type. If the apparatus requires transference between the digestion and distillation steps, this transfer must be carried out without loss. If the flask of the distillation apparatus is not fitted with a dropping funnel, add the sodium hydroxide immediately before connecting the flask to the condenser, pouring the liquid slowly down the side. | ||||||||||||||
8.2.If the digest solidifies, recommence the determination using a larger amount of sulphuric acid (3.4) than that specified above. | ||||||||||||||
8.3. For products with a low nitrogen content, the volume of sulphuric acid (3.6) to be placed in the collecting flask may be reduced, if necessary, to 10 or 15 ml and made up to 25 ml with water. | ||||||||||||||
GIVEN under my Official Seal, this 16th day of May, 1994. | ||||||||||||||
JOE WALSH, | ||||||||||||||
Minister for Agriculture, Food and | ||||||||||||||
Forestry. | ||||||||||||||
EXPLANATORY NOTE. | ||||||||||||||
These Regulations amend the European Communities (Marketing of Feedingstuffs) Regulations, 1984, (S.I. 200 of 1984) so as to give effect to Commission Directive 93/28/EEC which amends the method of analysis for the determination of crude protein in feedingstuffs. |