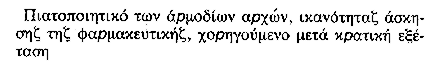S.I. No. 239/1987 - European Communities (Recognition of Qualifications in Pharmacy) Regulations, 1987.
S.I. No. 239 of 1987. | ||
EUROPEAN COMMUNITIES (RECOGNITION OF QUALIFICATIONS IN PHARMACY) REGULATIONS, 1987. | ||
The Minister for Health, in exercise of the powers conferred on him by section 3 of the European Communities Act, 1972 (No. 27 of 1972), hereby makes the following Regulations:— 1. These Regulations may be cited as the European Communities (Recognition of Qualifications in Pharmacy) Regulations, 1987. 2. (1) In these Regulations:— | ||
"the Council of the Society" means the Council of the Pharmaceutical Society of Ireland; | ||
"Council Directive 85/432/EEC" means the Council Directive so numbered and adopted by the Council of the European Communities on the 16th day of September 1985 and published in the Official Journal of the European Communities Volume 28 No. L253 dated 24 September 1985; | ||
"Council Directive 85/433/EEC" means the Council Directive so numbered and adopted by the Council of the European Communities on the 16th day of September 1985 and published in the Official Journal of the European Communities Volume 28 No. L 253 dated 24 September 1985; | ||
(2) In these Regulations, words and phrases shall have the same meaning as in the Council Directives referred to in paragraph (1). 3. The Pharmacy Act (Ireland), 1875 (38 & 39 Vict. c. 57) is hereby amended— | ||
( a ) by the addition in section 3 of the following definitions— | ||
"the term "Council Directive 85/432/EEC" means the Council Directive so numbered and adopted by the Council of the European Communities on the 16th day of September, 1985 and published in the Official Journal of the European Communities Volume 28 No. L 253 dated 24 September 1985; | ||
the term "Council Directive 85/433/EEC" means the Council Directive so numbered and adopted by the Council of the European Communities on the 16th day of September 1985 and published in the Official Journal of the European Communities Volume 28 No. L 253 dated 24 September 1985; | ||
the term "the period of the Greek derogation" means the period during which the Hellenic Republic makes use of the derogation provided by paragraph 1 of Article 3 of Council Directive 85/433/EEC." | ||
( b ) by the insertion of the following new section after section 22— | ||
"22A (1) Every person, being a national of a Member State of the European Economic Community, on making the prescribed application and on paying the prescribed fee and who at the time of such application holds any diploma, certificate or other evidence of formal qualification in pharmacy awarded in accordance with article 2 of Council Directive 85/432/EEC and specified in Schedule 2 to this Act shall be entitled under this Act to be registered as a pharmaceutical chemist. | ||
(2) Every person, being a national of a Member State of the European Economic Community, on making the prescribed application and on paying the prescribed fee and who at the time of such application holds a diploma, certificate or other evidence of formal qualification in pharmacy referred to in Article 6 of Council Directive 85/433/EEC and in respect of whom a certificate has been issued by a competent authority designated in accordance with Article 17 of the said Council Directive stating that he has been effectively and lawfully engaged in one of the activities (being an activity which is regulated in that State) referred to in paragraph 2 of Article 1 of Council Directive 85/432/EEC in a Member State for at least three consecutive years during the five years preceding the award of the certificate, shall be entitled under the Act to be registered as a pharmaceutical chemist. | ||
(3) Where by virtue of this section a name is registered, an indication that the name has been registered pursuant to this section shall be entered in the register against that name. | ||
(4) Where at any time during the period of the Greek derogation, the name of any person is registered in respect of the qualification specified in paragraph 5 of Schedule 2 to this Act or in respect of any other qualification in pharmacy awarded in the Hellenic Republic— | ||
(i) an indication that the registration is subject to the provisions of this subsection shall be entered in the register against that name; and | ||
(ii) the registration shall not authorise the person whose name is registered to do anything for which registration is required by any provision made by or under the Pharmacy Acts 1875 to 1977, the Misuse of Drugs Acts, 1977 and 1984 or the Poisons Acts, 1961 and 1977, except as an employed person in accordance with Council Regulation (EEC) No. 1612/68 adopted by the Council of the European Communities on the 15th day of October, 1968 and published in the Official Journal of the European Communities No. L 257 dated 19 October 1968 on freedom of movement for workers within the community. | ||
(5) In this section the term "prescribed" means prescribed by the Council of the Pharmaceutical Society of Ireland in regulations made with the approval of the Minister for Health under the Pharmacy Acts, 1875 to 1977." | ||
( c ) by the insertion after the Schedule to the Act of the following Schedule— | ||
"SCHEDULE 2 | ||
Diplomas, certificates and other evidence of formal qualifications in pharmacy. | ||
Belgium | ||
1. Le diplôme légal de pharmacien/het wettelijk diploma van apoteker (the legal diploma in pharmacy) awarded by the faculties of medicine and pharmacy of the Universities, by the Central examining board or by the State examining boards for university education. | ||
Denmark | ||
2. Bevis for bestået farmaceutisk kandidateksamen (the university pharmacy certificate). | ||
France | ||
3. The State diploma in pharmacy awarded by the universities or the State diploma of Doctor in Pharmacy awarded by the universities. | ||
Germany | ||
4. (1) Zeugnis über die staatliche Pharmazeutische Prüfung (the State examination certificate in pharmacy) awarded by the competent authorities. | ||
(2) Certificates from the competent authorities of the Federal Republic of Germany stating that the diplomas awarded after 8 May 1945 by the competent authorities of the German Democratic Republic are recognized as equivalent to those referred to in sub-paragraph (1) above. | ||
Greece | ||
| ||
(the certificate attesting competence to pursue the activity of a pharmacist) issued by the competent authorities following a State examination. | ||
Italy | ||
6. The diploma or certificate giving the right to practise pharmacy, obtained by passing a State examination. | ||
Luxembourg | ||
7. (1) The State pharmacy diploma awarded by the State | ||
Examining Board and signed by the National Minister of Education. | ||
(2) A diploma conferring on a national of the Grand Duchy a degree in respect of pharmacy which— | ||
( a ) has been granted otherwise than in a member State; | ||
( b ) is accorded official recognition by the National Minister for Education in accordance with the law of the Grand Duchy of 18th June 1969 on higher education and the recognition of foreign degrees and diplomas; and | ||
( c ) is approved for the purposes of section 22A of this Act by the Council of the Pharmaceutical Society of Ireland. | ||
The Netherlands. | ||
8. Het getuigschrift van met goed gevolg afgelegd apothekersexamen (the university pharmacy certificate). | ||
Portugal | ||
9. Carta de curso de licenciatura em Ciências farmêceuticas (the certificate in pharmaceutical sciences awarded by the universities). | ||
Spain | ||
10. Título de licenciado en farmacia (university degree in pharmacy awarded by the Ministry of Education and Science or by the universities). | ||
United Kingdom | ||
11. The certificate of Registered Pharmaceutical Chemist." | ||
4. The Pharmacy Act, 1962 (No. 14 of 1962) is hereby amended by the insertion in section 2 thereof of the following sub-section | ||
"3A An "authorised person" under this section shall not include a person registered by virtue of section 22A of the Pharmacy Act, 1875 where the shop for the dispensing or compounding of medical prescriptions or for the sale of poisons has been in operation for less than three years." | ||
5. The Council of the Society is hereby designated, pursuant to Article 17 of Council Directive 85/433/EEC, to be the competent authority to grant the certificate in the State for the purposes of paragraph (f) of Article 4 of that Directive and in granting the said certificate the Council of the Society shall satisfy itself that the requirements for such qualification set out in Article 2 of Council Directive 85/432/EEC have been complied with. | ||
6. The Council of the Society is hereby designated, pursuant to Article 17 of Council Directive 85/433/EEC, to be the competent authority in the State to issue and receive for the purposes of that Directive;— | ||
(i) certificates under Article 5; | ||
(ii) certificates under Article 6; | ||
(iii) certificates under paragraph 1 of Article 8; | ||
(iv) documents under paragraph 2 of Article 8; | ||
(v) information under paragraph 3 of Article 8; | ||
(vi) information under paragraphs 1 and 2 of Article 9; | ||
(vii) certificates under Article 10; | ||
(viii) information under Article 16. | ||
7. The Council of the Society— | ||
( a ) shall be the competent authority in the State in respect of the requirements to be fulfilled under paragraph 3 of Article 8 and under paragraph 2 of Article 9 of Council Directive 85/433/EEC; | ||
( b ) shall ensure the confidentiality of any information issued or received under Articles 8 and 9 of Council Directive 85/433/EEC. | ||
8. The Council of the Society may require of the competent authorities of another Member State confirmation of the authenticity of the diplomas, certificates and other evidence of formal qualifications issued in that other Member State and referred to in Chapters II and III of Council Directive 85/433/EEC, and also confirmation of the fact that the person concerned has fulfilled all the training requirements laid down in Council Directive 85/432/EEC. | ||
9. These Regulations shall come into operation on the 30th day of September, 1987. | ||
GIVEN under the Official Seal of the Minister for Health this 17th day of September, 1987. | ||
RORY O'HANLON, | ||
Minister for Health. | ||
EXPLANATORY NOTE | ||
The purpose of these Regulations, which come into force on 30th September, 1987, is to give statutory effect in this country to EEC Council Directives 85/432/EEC, 85/433/EEC and 85/584/EEC concerning the mutual recognition of diplomas, certificates and other evidence of formal qualifications in pharmacy in respect of nationals of Member States. | ||
The First Directive (No. 85/432/EEC) is concerned with setting and maintaining of the minimum standards to be observed in the training of pharmacists. | ||
The second Directive (No. 85/433/EEC as amended by Directive 85/584/EEC) concerns the mutual recognition of pharmacy qualifications awarded in the Member States and includes measures to facilitate the exercise of the right of persons holding such qualifications, with certain derogations, to practice their profession in any of the Member States. | ||
Articles 1 and 2 of the Regulations deal with definitions and other technical matters. | ||
Article 3 of the Regulations inserts a new Section and Schedule into the Pharmacy Act, 1875. This gives pharmacists with any of the qualifications specified in the Schedule the right to be registered in this country as Pharmaceutical Chemists. A special provision has been included to cater for existing pharmacists, and for current pharmacy students whose training may not satisfy the training requirements now laid down. | ||
It is also provided in pursuance of Article 3 of Directive 85/433/EEC, that during the period of the Greek derogation, registration by virtue of the Greek qualification is effective only when a person so registered is acting in an employed capacity. | ||
Article 4 inserts a new sub-section into Section 2 of the Pharmacy Act, 1962 . This provides, in pursuance of Article 2(2) of Directive 85/433/EEC, that a pharmacy in the State which has been in operation for less than 3 years shall not be managed or supervised by a pharmacist who has qualified by virtue of Section 22A of the Pharmacy Act, 1875. | ||
Article 5 designates the Council of the Pharmaceutical Society of Ireland as the body responsible for the award of the qualifications in pharmacy and for the maintenance of the required standards of training. | ||
Articles 6 and 7 designate the Council of the Pharmaceutical Society of Ireland as the competent authority or body in the State for the purpose of the functions listed in those Articles. | ||
Article 8 provides a means for the resolution of any doubts which may arise concerning qualifications. |