S.I. No. 42/1972 - Health (Antioxidant in Food) Regulations, 1972.
S.I. No. 42 of 1972. | |||||||||||||||||||||||
HEALTH (ANTIOXIDANT IN FOOD) REGULATIONS, 1972. | |||||||||||||||||||||||
The Minister for Health in exercise of the powers conferred on him by sections 5 , 54 and 59 of the Health Act, 1947 (No. 28 of 1947), sub-section (3) of section 38 of the Health Act, 1953 (No. 26 of 1953) and section 6 of the Health Act, 1970 (No. 1 of 1970) after consultation with the Minister for Industry and Commerce and the Minister for Agriculture and Fisheries hereby makes the following Regulations:— | |||||||||||||||||||||||
|
PART I. PRELIMINARY AND GENERAL. | |||||||||||||||||||||||
1. These Regulations may be cited as the Health (Antioxidant in Food) Regulations, 1972. | |||||||||||||||||||||||
2. These Regulations shall come into operation on the first day of September, 1972. | |||||||||||||||||||||||
3. (1) In these Regulations— | |||||||||||||||||||||||
"antioxidant" means any substance which delays, retards or prevents the development in food of rancidity or other deterioration of flavour due to oxidation but does not include lecithin, ascorbic acid or its salts or esters, tocopherols, citric acid, tartaric acid, phosphoric acid or any substance the use of which is permitted under Regulations, other than these Regulations, for the time being in force under Part V of the Health Act, 1947 ; | |||||||||||||||||||||||
"authorised officer" means an authorised officer for the purposes of Part IX of the Health Act, 1947 ; | |||||||||||||||||||||||
"butylated hydroxyanisole" means the substance conforming to the description, specifications and requirements for butylated hydroxyanisole contained in the Irish Pharmacopoeia; | |||||||||||||||||||||||
"butylated hydroxytoluene" means the substance conforming to the description, specifications and requirements for butylated hydroxytoluene contained in the Irish Pharmacopoeia; | |||||||||||||||||||||||
"container" includes any form of packaging of food and any wrapper or band; | |||||||||||||||||||||||
"dodecyl gallate" means the substance conforming to the description, specifications and requirements for dodecyl gallate contained in the Irish Pharmacopoeia; | |||||||||||||||||||||||
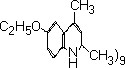
"ethoxyquin" means 1:2 dihydro-6-ethoxy-2:2:4-trimethylquinoline conforming to the specification relating thereto in the Second Schedule to these Regulations; | |||||||||||||||||||||||
"octyl gallate" means the substance conforming to the description, specifications and requirements for octyl gallate contained in the Irish Pharmacopoeia; | |||||||||||||||||||||||
"partial glycerol esters" means any compound formed by incompletely coterifying the hydroxyl groups of glycerol with either— | |||||||||||||||||||||||
( a ) any single fatty acid or | |||||||||||||||||||||||
( b ) any mixture of fatty acids or | |||||||||||||||||||||||
( c ) any mixture of fatty acids with one of the following organic acids—acetic acid, lactic acid, citric acid, tartaric acid, diacetyl tartaric acid or hydroxystearic acid, | |||||||||||||||||||||||
in which the proportion of sodium salt, if any, of any fatty acid present does not exceed two per cent of the partial glycerol ester; | |||||||||||||||||||||||
"permitted antioxidant" means any antioxidant described in the second column of the First Schedule to these Regulations and, in the case of ethoxyquin, which complies with the specification for that antioxidant in the Second Schedule to these Regulations; | |||||||||||||||||||||||
"pre-packed" means made up in a container for the purpose of retail sale prior to exposure or offer for sale; | |||||||||||||||||||||||
"propyl gallate" means the substance conforming to the description, specifications and requirements for propyl gallate contained in the Irish Pharmacopoeia; | |||||||||||||||||||||||
"specified food" means any food of a description specified in the first column of the First Schedule to these Regulations. | |||||||||||||||||||||||
(2) References in these Regulations to percentages and parts per million shall be construed as references to percentages and parts per million calculated by weight. | |||||||||||||||||||||||
(3) Any reference in these Regulations to a label borne on a container shall be construed as including a reference to any legible marking on the container however effected. | |||||||||||||||||||||||
4. Any reference in these Regulations to an owner or to a person in apparent charge or control of food shall in the case of food purchased from an automatic machine be construed as a reference— | |||||||||||||||||||||||
( a ) where the name and address of the proprietor is stated on the machine and such address is in the State, to the proprietor of the machine; | |||||||||||||||||||||||
( b ) in other cases, to the occupier of the premises at or on which the machine stands or to which it is affixed. | |||||||||||||||||||||||
5. These Regulations shall not apply to any food which is intended to be exported or re-exported. | |||||||||||||||||||||||
6. These Regulations shall be enforced and executed by health boards in their functional areas. | |||||||||||||||||||||||
7. (1) Where a sample of any food has been certified under the Health (Sampling of Food) Regulations, 1970 ( S.I. No. 50 of 1970 ) not to comply with these Regulations, an authorised officer may seize, remove and detain such food as being food which is unfit for human consumption. | |||||||||||||||||||||||
(2) With the consent in writing of the owner or person in apparent charge or control of such food an authorised officer may destroy or otherwise dispose of it so as to prevent its use for human consumption. | |||||||||||||||||||||||
(3) An authorised officer who has seized any food in pursuance of the provisions of this article may, on giving notice in writing to the owner or person in apparent charge or control of such food of his intention to do so, apply to a Justice of the District Court for an order directing that such food be destroyed or otherwise disposed of as being food which is unfit for human consumption. | |||||||||||||||||||||||
(4) A Justice of the District Court to whom the application is made for an order under sub-article (3) of this article shall, if satisfied that such food does not comply with these Regulations, order that it be destroyed or otherwise disposed of after such period, not exceeding fourteen days, as may be specified in such order, as being food which is unfit for human consumption and an authorised officer shall destroy or dispose of it accordingly. | |||||||||||||||||||||||
8. A person shall give to any authorised officer all reasonable assistance that the officer may require in the performance of his duties under these Regulations and such assistance shall include the giving of information relating to the composition and use of any food and the identity of the person from whom or the place from which any such food has been obtained and the person to whom and the place to which it has been consigned or the manner in which it has otherwise been disposed of. | |||||||||||||||||||||||
|
PART II. SALE ETC. OF ANTIOXIDANT AND OF FOOD CONTAINING ANTIOXIDANT. | |||||||||||||||||||||||
9. Subject to the provisions of these Regulations a person shall not import, distribute, sell or expose for sale | |||||||||||||||||||||||
( a ) any antioxidant, other than a permitted antioxidant, for use in the manufacture or preparation of food; | |||||||||||||||||||||||
( b ) any permitted antioxidant in such a manner as to be likely to lead to its use contrary to these Regulations. | |||||||||||||||||||||||
10. Subject to the provisions of these Regulations, a person shall not import, distribute, sell or expose for sale any food which has in it or on it any added antioxidant. | |||||||||||||||||||||||
11. (1) Any specified food may have in it or on it an amount not exceeding that specified in the third column of Part I and Part II of the First Schedule to these Regulations of an antioxidant described in the second column of the said Parts in relation to such food. | |||||||||||||||||||||||
(2) Where under the provisions of sub-article (1) of this article a specified food may have in it or on it butylated hydroxyanisole or butylated hydroxytoluene or mixtures thereof within the limits specified for them in Part I of the First Schedule to these Regulations, butylated hydroxyanisole or butylated hydroxytoluene or mixtures thereof may be used in conjunction with propyl gallate or octyl gallate or dodecyl gallate or mixtures thereof within the limits specified for them in Part I of the said Schedule, provided that the total amount of antioxidant shall not exceed, in the case of specified foods in items a and b of the said Part I, 300 parts per million, and in the case of specified foods in item c of the said Part I, 1,000 parts per million. | |||||||||||||||||||||||
(3) Notwithstanding sub-article (1) of this article a person shall not import, distribute, sell or expose for sale any food which has in it or on it any added antioxidant and which is recommended in any mark or label on or attached to it or on or attached to its container to be for consumption by babies or infants. | |||||||||||||||||||||||
(4) Any food which contains any specified food may contain an amount of the antioxidants not exceeding that permitted by these Regulations for such specified food. | |||||||||||||||||||||||
|
PART III.LABELLING AND ADVERTISING. | |||||||||||||||||||||||
12. (1) Subject to the provisions of these Regulations a person shall not import, distribute, sell or expose for sale any food mentioned in the first column of Part I or of Part II of the First Schedule to these Regulations which has in it or on it any permitted antioxidant in respect of such food unless it is packed in a container bearing a label in one of the forms specified for such a label in the Third Schedule to these Regulations or in the case of a retail sale of food which is not pre-packed a notice to the effect that the food contains antioxidant is exhibited in a conspicuous place so as to be easily readable by a purchaser of such food. | |||||||||||||||||||||||
(2) Each label to which sub-article (1) of this article relates shall be securely affixed to or be part of the container and shall be so placed as to be clearly visible and if the container bears a main label shall be placed in close proximity thereto or be part thereof. | |||||||||||||||||||||||
(3) Where in accordance with sub-article (1) of this article, a container is required to bear a label and such container is wrapped in paper or any other wrapper through which the label on the container is not clearly readable, the outermost wrapper shall on any exposure or offer for sale by retail bear a label as if it were the container to which that sub-article applies. | |||||||||||||||||||||||
(4) The provisions of this article shall not apply to— | |||||||||||||||||||||||
( a ) a sale in the course of a catering business of any specified food for immediate consumption, or | |||||||||||||||||||||||
( b ) a sale by retail of apples and pears which have in them or on them ethoxyquin. | |||||||||||||||||||||||
13. (1) A person shall not import, distribute, sell or expose for sale any substance for use as an antioxidant in food unless such substance is packed in a container bearing a label in the form specified for such a label in the Third Schedule to these Regulations. | |||||||||||||||||||||||
(2) Each label to which sub-article (1) of this article relates shall be securely affixed to or be part of the container and shall be so placed as to be clearly visible and if the container bears a main label shall be placed in close proximity thereto or be part thereof. | |||||||||||||||||||||||
14. (1) A person shall not advertise for sale— | |||||||||||||||||||||||
( a ) any antioxidant for use in food other than an antioxidant which is a permitted antioxidant in respect of such food, | |||||||||||||||||||||||
( b ) any food containing a permitted antioxidant in such a manner as to indicate or imply that the food is intended mainly for consumption by babies or infants, | |||||||||||||||||||||||
( c ) any permitted antioxidant for use in food or any food containing a permitted antioxidant in such a manner as to be likely to lead to its use or sale contrary to these Regulations. | |||||||||||||||||||||||
(2) Where a person is charged with a contravention of this article, it shall be a good defence to show that the advertisement was published in such circumstances that he did not know and could not, by the exercise of reasonable care, have known that he was taking part in the publication of such advertisement. | |||||||||||||||||||||||
|
FIRST SCHEDULE. | |||||||||||||||||||||||
FOODS WHICH MAY HAVE IN THEM OR ON THEM ADDED ANTIOXIDANT AND THE DESCRIPTIONS AND AMOUNTS OF ANTIOXIDANT WHICH MAY BE ADDED IN EACH CASE. | |||||||||||||||||||||||
PART I. | |||||||||||||||||||||||
| |||||||||||||||||||||||
PART II. | |||||||||||||||||||||||
| |||||||||||||||||||||||
|
SECOND SCHEDULE. | |||||||||||||||||||||||
SPECIFICATION FOR ETHOXYQUIN. | |||||||||||||||||||||||
| |||||||||||||||||||||||
|
THIRD SCHEDULE. | |||||||||||||||||||||||
1. The label to which sub-article (1) of article 13 relates shall be in one of the following forms:— | |||||||||||||||||||||||
CONTAINS ANTIOXIDANT | |||||||||||||||||||||||
or | |||||||||||||||||||||||
CONTAINS PERMITTED ANTIOXIDANT | |||||||||||||||||||||||
2. The label to which sub-article (1) of article 14 relates shall be in the following form or in a form substantially to the like effect:— | |||||||||||||||||||||||
This antioxidant contains | |||||||||||||||||||||||
(X) | |||||||||||||||||||||||
(Y) | |||||||||||||||||||||||
There shall be inserted at (X) in every such declaration a true statement of the percentage, or the number of parts per million, by weight in figures, excluding fractions, correct to the nearest whole digit, or in words and figures excluding fractions, correct to the nearest whole digit, of each and every antioxidant present in the preparation in the container and a correct description of each antioxidant to which such statement relates. There shall be inserted at (Y) a correct description of any other substance present in the preparation in the container and where more than one such substance is present such substances shall be declared in the order of the proportion in which they were present at the time of sale by the manufacturer, the substance present in the greatest proportion by weight being specified first. | |||||||||||||||||||||||
3. Each label prescribed in this Schedule shall be printed distinctly and legibly in dark type upon a light-coloured ground or in light type upon a dark-coloured ground. The type used for labels on containers of quantities of not less than 4 ounces or 4 fluid ounces, as the case may be, shall be not less than one-eighth of an inch in height, and the type used for labels on containers of quantities of less than 4 ounces or 4 fluid ounces, as the case may be, shall be not less than one-sixteenth of an inch in height. Every letter of every word shall be of uniform size and colour provided that the initial letter in any word may be larger than the other letters in that word. | |||||||||||||||||||||||
GIVEN under the Official Seal of the Minister for Health, | |||||||||||||||||||||||
this 17th day of February, 1972. | |||||||||||||||||||||||
ERSKINE H. CHILDERS, | |||||||||||||||||||||||
Minister for Health. | |||||||||||||||||||||||
EXPLANATORY NOTE. | |||||||||||||||||||||||
These Regulations, which come into operation on 1st September, 1972, provide that food being imported, distributed, sold or exposed for sale shall not have in it or on it antioxidant, as defined, except as provided for in the Regulations and prescribe the foods which may contain antioxidant and the amount of such antioxidant that may be contained in them. | |||||||||||||||||||||||
The Regulations prohibit the importation, distribution, sale or exposure for sale for use in food of any antioxidant other than a permitted antioxidant or any permitted antioxidant in such a manner as to be likely to lead to its use contrary to the Regulations. | |||||||||||||||||||||||
The marking, labelling or advertising of any food as food intended for babies or infants is prohibited if the food has any added antioxidant in or on it. The Regulations also provide for the labelling of specified foods containing antioxidants and of antioxidants. | |||||||||||||||||||||||
The Regulations provide that where a sample of food has been certified not to comply with the Regulations, an authorised officer may seize, remove and detain such food as being food which is unfit for human consumption and, in certain circumstances, destroy it. |