S.I. No. 155/1949 - Standard Specification (White Pigments For Paints) Order, 1949.
S.I. No. 155 of 1949. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
STANDARD SPECIFICATION (WHITE PIGMENTS FOR PAINTS) ORDER, 1949. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
I, DANIEL MORRISSEY, Minister for Industry and Commerce, in exercise of the power conferred on me by subsection (3) of section 20 of the Industrial Research and Standards Act, 1946 (No. 25 of 1946), hereby order as follows : 1. This Order may be cited as the Standard Specification (White Pigments for Paints) Order, 1949. 2.—(1) The specification set forth in Part II of the Schedule to this Order is hereby declared to be the standard specification for the commodity described in Part I of the said Schedule. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) The said standard specification may be cited as Irish Standard 21 : 1949. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
SCHEDULE. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PART I. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
WHITE PIGMENTS FOR PAINTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PART II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In this specification, the letters I.S., when followed by two sets of numbers, refer to the Irish Standard of which the first is the serial number and the second the year of its promulgation by the Minister for Industry and Commerce. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In this specification, the letters B.S., when followed by two sets of numbers, refer to the British Standard of which the first is the serial number and the second is the year of its publication by the British Standards Institution. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SCOPE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. This specification applies to the following materials suitable for use as white pigments for paints. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) White lead. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) Basic sulphate of lead. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) Zinc oxide type 1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) Zinc oxide type 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(e) Lithopone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(f) Antimony oxide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(g) Titanium dioxide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(h) Titanium white type 1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(j) " " type 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(k) " " type 3. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(l) " " type 4. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(m) " " type 5. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(n) " " type 6. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(p) " " type 7. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(q) " " type 8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PHYSICAL CONDITION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
2. White pigments shall be either in the form of a soft, dry powder, or in a condition such that they may be readily crushed to that form with a palette knife. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COMPOSITION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3. Each white pigment, after drying at 100°±2°C., shall conform in composition to the provisions set out for it below. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) White lead shall contain not less than 99·0 per cent. of hydroxy-carbonate of lead. The hydroxy-carbonate of lead shall consist of not less than 20·0 per cent. and not more than 33·0 per cent. of lead hydroxide, and of not more than 80·0 per cent. and not less than 67 per cent. of normal carbonate of lead. The lead hydroxide and the lead carbonate shall be chemically combined. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) Basic sulphate of lead shall contain not less than 94 per cent. of basic sulphate of lead, not more than 5 per cent. of zinc oxide and not more than 1 per cent. of other materials. The basic sulphate of lead shall consist of not less than 20 per cent. and of not more than 40 per cent. of lead monoxide, and of not more than 80 per cent. and not less than 60 per cent. of normal sulphate of lead. The lead monoxide and the normal sulphate of lead shall be chemically combined. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) Zinc oxide, type 1, shall contain not less than 99 per cent. zinc oxide and not more than 0·2 per cent. of lead compounds, expressed as the element lead. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(d) Zinc oxide, type 2, shall contain not less than 96 per cent. zinc oxide and not more than 3 per cent. lead compounds expressed as the element lead. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(e) Lithopone shall consist of calcined co-precipitated barium sulphate and zinc sulphide, free from separate addition of either of these materials. It shall contain not less than 99 per cent. of barium and zinc compounds, of which not more than 32 per cent, and not less than 26 per cent. shall consist of zinc sulphide, not more than 2 per cent. shall consist of zinc oxide, and the remainder shall consist of barium sulphate. For the purposes of this specification, strontium sulphate may replace barium sulphate in lithopone, provided that the total amount of strontium sulphate does not exceed 5 per cent. of the total weight of the lithopone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The amount of zinc sulphide shall be determined by the method described in Appendix A. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(f) Antimony oxide shall contain not less than 99 per cent. of antimony oxide (Sb2O3). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(g) Titanium dioxide shall contain not less than 97 per cent. of titanium dioxide (TiO2). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(h) Titanium white, types 1, 2, 3, and 4 shall consist mainly of titanium dioxide and precipitated barium sulphate. They shall be free from barytes and shall contain not more than 5 per cent. of material other than titanium dioxide and barium sulphate. Each shall contain a proportion of titanium dioxide not less than that set out for it below. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(i) Titanium white, type 5, shall contain not less than 25 per cent. of titanium dioxide (TiO2) and not less than 50 per cent. of precipitated barium carbonate (BaCO3). It may contain precipitated barium sulphate. It shall be free from witherite and barytes and shall contain not more than 5 per cent. of material other than titanium dioxide, precipitated barium carbonate and precipitated barium sulphate. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The material shall closely resemble an agreed sample in the shape and size of its particles. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(j) Titanium white type 6, shall consist substantially of titanium dioxide of the rutile crystal structure in combination with antimony oxide or zinc oxide or both, and shall be free from separate addition of these or other materials. It shall ,contain not less than 85 per cent. titanium dioxide, and not less than 97 per cent. of titanium dioxide and antimony oxide and/or zinc oxide. When tested by the methods described in Appendices B and C the pigment shall not show the presence of free zinc oxide or free antimony oxide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(k) Titanium white, types 7 and 8, shall consist of titanium white, type 6, modified for improved resistance to chalking, and barytes (BaSO4) complying with I.S. 19 : 1949 or barium carbonate (BaCO3) complying with B.S. 260 : 1938. When tested by the methods described in Appendices B and C, the pigment shall not show the presence of free zinc oxide or free antimony oxide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Titanium white, type 7, shall contain not less than 25 per cent. of titanium white, type 6, and not less than 71·5 per cent of barium sulphate (BaSO4). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Titanium white, type 8, shall contain not less than 25 per cent. of titanium white, type 6, and not less than 72·5 per cent. of barium carbonate (BaCO3). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COLOUR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4. The colour of each white pigment shall closely match that of an agreed sample when compared with it in the manner described in Appendix D. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
When tested for stability of colour in the manner described in Appendix E, the colour of lithopone shall be not darker than that of an agreed sample. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COARSE PARTICLES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. When tested in the manner described in Appendix F, the content of coarse particles of each white pigment shall be not greater than the amount set out for it below. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PARTICLE SIZE OF ZINC OXIDE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
6. The particle size of zinc oxide shall be such that it shall all pass through a No. 100 test sieve conforming to B.S. 410 : 1943. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
VOLATILE CONTENT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
7. The loss in weight of basic sulphate of lead on heating to constant weight at 100°±2°C. shall not exceed 0·7 per cent. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The loss in weight of the other white pigments on heating to constant weight at 100°±2°C. shall not exceed 0·5 per cent. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OIL ABSORPTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
8. With the exception of zinc oxide type 1 and of zinc oxide type 2, the oil absorption number of each white pigment when determined in the manner described in Appendix G shall be within the limits set out for it in Table 1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zinc oxide type 1 and zinc oxide type 2 shall be either of low oil absorption, which shall be designated as Grade L, or high oil absorption, which shall be designated as Grade H. The oil absorption number of these white pigments, when determined in the manner described in Appendix G. shall be : | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
for zinc oxide type 1, or type 2, grade L, not less than 14 and not more than 21, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
for zinc oxide type 1, or type 2, grade H, not less than 21 and not more than 27. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
REDUCING POWER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
9. The reducing power of the white pigment shall be not inferior to that of an agreed sample, when compared in the manner described in Appendix H. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
RESISTANCE TO CHALKING OF TITANIUM WHITE, TYPES 6, 7 AND 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
10. When a paint film incorporating the white pigment is tested in the manner described in Appendix K the time taken for the commencement of chalking shall not be significantly less than that of a paint film incorporating an agreed sample tested in the same manner and at the same time. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
MATTER SOLUBLE IN WATER, ACIDITY, ALKALINITY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
11. The percentage of matter soluble in water, and the acidity or alkalinity of the aqueous extracts determined by the methods described in Appendix L, shall be not greater than the values set out for each white pigment in Table 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SAMPLING AND SIZE OF SAMPLE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
12. Representative samples each weighing not less than 1 lb., shall be taken in triplicate from one or more original and previously unopened containers, or from the bulk during filling. They shall be packed in clean dry airtight non-absorbent containers made of material on which the sample has no action. The containers shall be of a size such that they are nearly filled by the sample. Each container so filled shall be sealed and shall be marked with the date of sampling, and with sufficient information to identify the sample. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AGREED SAMPLE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
13. Except where otherwise agreed between the purchaser and the vendor, the agreed sample referred to in this specification shall comply in all respects with the requirements of this specification. The sample shall weigh not less than 1 lb. and shall be packed in the manner described in Clause 12. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
MATERIALS USED IN TESTING SAMPLES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
14. Where linseed oil, turpentine, or white spirit are used in carrying out the tests described in the Appendix they shall conform respectively to I.S. 14 : 1949, I.S. 12 : 1949 and I.S. 11 : 1949. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Test sieves used in carrying out the tests described in the Appendices shall be test sieves conforming to B.S. 410 : 1943. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX A. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the determination of Zinc Sulphide in Lithopone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Half a gram of the white pigment shall be gently heated with dilute hydrochloric acid (6N) in an atmosphere of hydrogen and the hydrogen sulphide evolved shall be passed into an ammoniacal solution of cadmium chloride by means of a stream of hydrogen. The hydrogen used shall be freed from hydrogen sulphide by passing it through an alkaline solution of lead nitrate. The gas discharged from the ammoniacal solution of cadmium chloride shall cause no reaction when passed through a second ammoniacal solution of cadmium chloride. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The cadmium sulphide precipitated from the ammoniacal solution of cadmium chloride shall be treated for two minutes with 10 ml. of water, 10 ml. of concentrated hydrochloric acid and 50 ml. of N/10 iodine solution in a stoppered flask which is shaken occasionally. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The excess iodine present shall be titrated with N/10 sodium thiosulphate solution using starch as indicator and the quantity of zinc sulphide present in the white pigment determined on the basis of 1 ml. of N/10 sodium thiosulphate solution being equivalent to 0·004879 g. of zinc sulphide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The ammoniacal solution of cadmium chloride used shall be prepared by dissolving 20·1g. of crystalline cadmium chloride (CdCl22½H2O) in 100 ml. of water and adding 100 ml. of ammonia (sp. gr. 0·880) and then mixing and adding 900 ml. of water. The solution shall then be mixed thoroughly and filtered if it appears cloudy. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
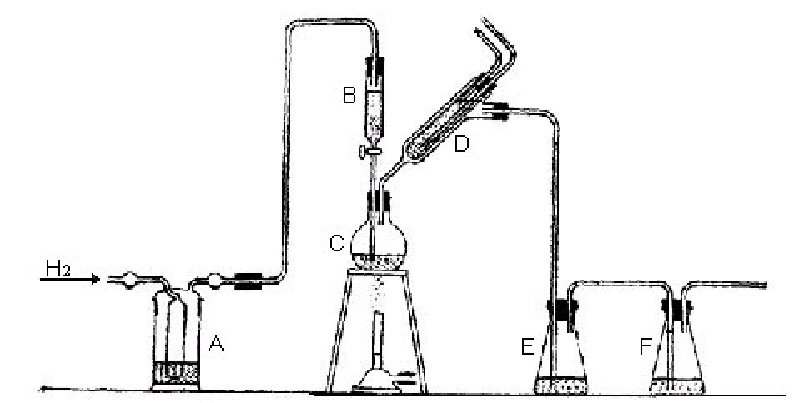
A form of apparatus suitable for carrying out the determination is shown in Fig. 1. The procedure using this form of apparatus is as follows : | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Weigh 0·5 g. of the white pigment and place in flask C, Place 50 ml. of the ammoniacal cadmium chloride solution, 30 ml. of water and a few drops of strong ammonia in conical flask E and place the same quantity of a similar mixture in conical flask F. Connect up the apparatus as shown in Fig. 1 and pass a current of hydrogen through the apparatus until the issuing gas, when tested, shows no trace of air. Fill the dropping funnel B with dilute hydrochloric acid (6N) and connect up the apparatus again. With the hydrogen still running, open the tap of the dropping funnel and allow the acid to drop slowly into flask C. When B is empty gently heat flask C until the liquid boils and the evolved H2S is swept through the apparatus by the stream of hydrogen. After the liquid in the flask C has boiled gently for five minutes allow the apparatus to cool, the current of hydrogen being continued all the time. The liquid in flask F should be quite clear unless some of the H2S has escaped from flask E by too rapid evolution of the gas. Filter the contents of flask E, and wash the residue once with cold water. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Place 10 ml. of water and 10 ml. of concentrated hydrochloric acid in an empty flask and then add 50 ml. of N/10 iodine solution and drop in the filter paper and residue. Stopper the flask and allow to stand for two minutes, shaking occasionally, and then titrate the excess iodine with N/10 sodium thiosulphate solution using starch as indicator. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
One ml. of N/10 sodium thiosulphate solution = 0·00487 g. ZnS. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX B. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the detection of free Zinc Oxide in Titanium White, Types 6, 7, and 8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The white pigment when made into a paint using a medium having an acid value not greater than 35, and tested by the method given below shall not show an increase in viscosity greater than that of an intimate mixture of titanium dioxide (conforming to this specification), and 0·5 per cent. of zinc oxide type 1, grade L (conforming to this specification), when made into a paint using the same medium and tested in the same way. Suitable media for making the paints are : | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(a) Short oil natural congo resin varnish with an oil length of nine parts of resin and seven parts of oil. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) A mixture of linseed oil and oleic acid. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The medium used shall be agreed to by the purchaser and the vendor. The test shall be carried out as follows : | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The paints to be tested shall be made by mixing the white pigment (50 g.) with the medium (80 g.) and passing through a laboratory cone mill, using sufficient white spirit to facilitate mixing and to yield paints of suitable consistency for the test. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The paints shall be placed in air-tight containers and maintained at a temperature of 40° C. for 48 hours. They shall then be allowed to cool to room temperature and, after thorough mixing, the viscosity of each paint at 25° C. shall be determined. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A suitable apparatus for determining the viscosity is the Ford Cup No. 4 manufactured by the Ford Motor Co., Dagenham, England. Using this apparatus a suitable consistency for the test is obtained by addition of white spirit until the reading of the apparatus at 25° C. lies between 60 to 80 seconds in the case of medium (a) and 30 to 50 seconds in the case of medium (b). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX C. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the detection of free Antimony Oxide in Titanium White Types 6, 7 and 8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The white pigment (2 g.) shall be suspended in N. hydrochloric acid solution (25 ml.) at room temperature and a steady stream of hydrogen sulphide gas passed through the suspension for five minutes. The development of a colour greater in intensity than Colour No. 359, Middle Buff, specified in B.S. 381c : 1948, shall be regarded as indicating the presence of free antimony oxide in the pigment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX D. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the Comparison of Colour. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The white pigment shall be rubbed down to a smooth mixture with refined linseed oil. Additional refined linseed oil shall be thoroughly incorporated in sufficient quantity to give a mixture which can be evenly spread on a smooth surface. The agreed sample shall be treated similarly and at the same time. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The two mixtures shall be spread in the same direction in alternate strips on a clear colourless glass plate. These strips shall be not less than ¾ in. wide and shall have touching edges not less than 1½ in. long. The strips shall be compared in diffused daylight immediately after application. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX E. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method of test for stability of Colour of Lithopone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The white pigment shall be ground with a muller to paint consistency, using a mixture of 4 parts by volume of boiled linseed oil to 1 part by volume of turpentine or white spirit, and the mixture painted out on a clear glass plate and allowed to dry. A second coat shall be applied and allowed to dry. The plate shall then be exposed for a week in a vertical position in a well-ventilated room maintained at a temperature of 16° C. to 21° C. and so arranged that its surface shall be illuminated by diffused daylight for at least 6 hours during each day of the testing period. In order to avoid interference through excessive humidity, care shall be taken that throughout the drying process the temperature of the room is above the dewpoint. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The agreed sample shall be tested by the same method and at the same time and the colours of the two samples compared after the exposure. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX F. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the determination of Coarse Particles. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The quantity of each white pigment to be used in the determination of coarse particles shall be : | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The appropriate quantity of white pigment shall be placed on a plate of glazed porcelain, ground glass or marble. Refined linseed oil shall be added and rubbed in thoroughly with a palette knife until a smooth paste is obtained. In the case of titanium white, type 5, the rubbing out shall be carried out for 10 minutes using a broad palette knife or glass muller. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The paste shall be mixed with 100 ml. of white spirit and the mixture transferred by washing with white spirit to a No. 240 test sieve conforming to B.S. 410 : 1943. The residue remaining on the sieve shall be washed with white spirit, not under pressure, and gently brushed with a soft camel-hair pencil until the washings are clear. The final residue shall be dried for one hour in an oven at 100°±2°C. and weighed. The residue calculated as a percentage of the original sample shall be taken as the percentage of coarse particles. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In determining the content of the coarse particles of zinc oxide, the zinc oxide before being washed through the No. 240 test sieve shall be washed through a No. 100 test sieve conforming to B.S. 410 : 1943 and the residue thereon treated as above. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX G. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the determination of Oil Absorption. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The quantity of white pigment to be used in the determination of oil absorption shall be : | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The appropriate quantity of white pigment shall be placed on a plate of glazed porcelain or ground glass, or marble. Refined linseed oil shall be added gradually and rubbed thoroughly into the whole of the pigment with a palette knife until a coherent mass is obtained. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The rate of addition of the linseed oil shall be regular and the incorporation of the oil with the pigment shall occupy 20 minutes. The weight of the oil used shall be noted and the oil absorption number of the white pigment shall be calculated as the weight of oil required by 100 g. of the white pigment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The oil absorption number of the agreed sample, if any, shall be determined in a similar manner and at the same time. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX H. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the comparison of reducing power. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Twenty grams of basic sulphate of lead or ten grams of other white pigments shall be made into a smooth stiff paste with refined linseed oil. Vegetable black (0·1 g.) conforming to B.S. 286 : 1937, shall be thoroughly rubbed out with refined linseed oil on a glass slab for five minutes, using a steel palette knife. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The black-in-oil and the white pigment paste shall then be thoroughly incorporated and rubbed out on a glass slab, until no further change in colour is manifest and the whole has been formed into a smooth paste of consistency such that it is capable of being evenly spread on a smooth surface. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A mixture shall be prepared from the agreed sample by treating it in the same way and at the same time as above, and reducing it to the same apparent consistency by the addition of refined linseed oil. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The two mixtures shall be spread in the same direction in alternate strips on a clear colourless glass plate. These strips shall be not less than ¾ in. wide and shall have touching edges of not less than 1½ in. in length. The shade and intensity of the colour of the strips shall be compared in diffused daylight immediately after application. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX K. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for testing resistance to chalking of Titanium White Types 6, 7 and 8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Two paints of similar constituent proportions shall be prepared at the same time and in the same manner, one containing the titanium pigment under test and the other containing the agreed sample of titanium pigment using an oil or other agreed medium. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The two paints shall be applied vertically side by side on the same panel which shall be of wood or other agreed material and given identical unsheltered exposure in the open air facing south at an inclination of 45°. The area of each paint exposed shall be not less than 18 sq. in. The time taken for chalking to commence shall be determined by regular and frequent observations of the panel by the following method : | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A disc of soft rubber 1 in. in diameter and 1/8 in. thick shall be placed on a piece of black cloth (such as velvet, Silesian or Italian) 1 in. by 4 in. and a weight of 100 g. shall be placed on the rubber disc. The cloth carrying the disc and weight shall then be drawn for a distance of 2 in. in approximately one second over the surface of the paint while the panel rests in a horizontal position. When the surface of the black cloth is just perceptibly marked white, chalking shall be regarded as having commenced. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
APPENDIX L. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Method for the determination of Matter Soluble in Water, Acidity, Alkalinity. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Five grams of the white pigment shall be wetted thoroughly with 2 ml. of ethyl alcohol and then boiled for five minutes with 200 ml. of neutral distilled water. The mixture shall then be cooled to room temperature, made up to 250 ml. with neutral distilled water, shaken and filtered. After rejection of the first 50 ml., a measured quantity of the filtrate shall be evaporated to dryness. The residue so obtained shall be dried to constant weight in an oven maintained at a temperature of 100°±2°C. This weight expressed as a percentage of the original weight of the pigment shall be taken as the percentage of matter soluble in water. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The acidity or alkalinity shall be determined by titrating further aliquot portions of the filtrate using methyl red as indicator. The acidity shall be calculated on the material as the percentage equivalent of sulphuric acid (H2SO4). The alkalinity shall be calculated on the material as the percentage equivalent of sodium carbonate (Na2CO2). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GIVEN under my Official Seal, this 23rd day of December, 1949. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DANIEL MORRISSEY, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Minister for Industry and Commerce. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A. Contains alkaline lead nitrate to remove H2S from the hydrogen. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
B. Is a dropping funnel holding 50 ml. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
C. Is a round-bottomed flask of 250 cc. capacity. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
D. Is a short Cribb's condenser. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
E. Contains 50 ml. of ammoniacal cadmium chloride solution. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
F. Contains ammoniacal cadmium chloride solution. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fig 1. Apparatus for determining sulphide in lithopone. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||